By Marisabel Caballero, Global Technical Manager Poultry
As the planet’s climate experiences changes, new patterns affect the microbial communities colonizing crops. Recently, several areas of the planet have experienced extreme temperatures, drought, changes in the humid/dry cycles, and an increase in atmospheric carbon dioxide (1,2). As a response, the fungi affecting the crops have shifted their geographical distribution, and with this, the pattern of mycotoxin occurrence also changed. For instance, in Europe, we are looking at higher frequencies and levels of Aflatoxins (AF), Ochratoxins (OT), and Fumonisins (FUM) than ten or even five years ago (2-4).
This affects animal production, as mycotoxin challenges show increased frequency, quantity, and variety. Mainly long-living animals, such as laying hens and breeders, can have a higher risk. Moreover, mycotoxins can also be carried over to the eggs, potentially risking human health in the case of layers (table eggs) and in the case of breeder hens, hatchery performance and day-old chick (DOC) quality.
Laying hens and breeders: carryover of mycotoxins into eggs
Most mycotoxins are absorbed in the proximal part of the gastrointestinal tract (Table 1). This absorption can be high, as in the case of aflatoxins (~90%), but also very limited, as in the case of fumonisins (<1%), with a significant portion of unabsorbed toxins remaining within the lumen of the gastrointestinal tract (5).
Once mycotoxins are ingested, detoxification and excretion processes are started by the body, and at the same time, organ damage ensues. The detoxification of mycotoxins is mainly carried out by the liver (6), and their accumulation happens primarily in the liver and kidneys. However, accumulation in other tissues, such as the reproductive organs and muscles, has also been found (7-9). The detoxification process’ objective is the final excretion of the toxins, which occurs through urine, feces, and bile; often, the toxins can also reach the eggs (7-20).
Table 1: mycotoxin absorption rates for poultry and their carry-over rate into eggs
| Mycotoxin | Main absorption sites | Absorption rate in poultry | Carry-over rate into eggs |
| Aflatoxins | Duodenum, jejunum | ≈90% | ≈0.55% |
| DON | Duodenum, jejunum | ≈20% | ≈0.001% |
| Fumonisins | Duodenum, jejunum | ≈1% | ≈0.001% |
| Ochratoxin | Jejunum | ≈40% | ≈0.15% |
| T-2 | Duodenum, jejunum | ≈20% | ≈0.10% |
| Zearalenone | Small & large intestine | ≈10% | ≈0.30% |
(Adapted from 5, 7-17, 19-21)
Table 1 shows carry-over rates of mycotoxins into eggs, resulting from diverse studies (7-10, 14, 16, 19). However, the same studies indicate that results can vary broadly due to different factors, as reviewed by Völkel and collaborators (26). This variability is related to the amount and source of contamination, way of application, period, and the possible co-occurrence of various mycotoxins or several metabolites. Other factors to consider are animal-related, such as species, breed, sex, age group, production level, and health status. Environmental and management factors can play a role in carry-over rates, and finally, detection limits and analytical procedures also influence these results. In summary, highly varying carry-over has been demonstrated, and the risk needs to be considered when animals are exposed.
Mycotoxins in breeder’s feed impact hatchery performance and day-old chick quality
When hens are exposed to mycotoxins, their effects on the intestine, liver, and kidney decrease egg production and quality (10, 14, 27), and, in the case of breeders, consequently, affect hatchery performance, DOC production, and DOC quality (28-30). The main effects of mycotoxins, when we speak about DOC production, are exerted in the gastrointestinal tract, the liver, and the kidneys, affecting embryos and young chicks:
- Intestine and kidneys: Mycotoxins harm the intestinal epithelium and have nephrotoxic effects, affecting calcium and vitamin D3 absorption and metabolism, necessary for eggshell quality (31). Thin and fragile shells can increase embryonic mortality, lower embryonic weight gain, and hinder hatchability (32).
- Liver: The liver plays a central role in egg production as it is responsible for vitamin D3 metabolism, the production of nutrient transporters, and the synthesis of the lipids that make up the yolk. Thus, when liver function is impaired, the internal and external quality of the egg declines, which affects DOC production (31-34).
- Embryo and young chicks: Studies (33-38) have found how mycotoxins affect the embryos. In general, there are two possibilities: the direct one, when the mycotoxin is transferred into the egg, and the indirect one, when the mycotoxin impacts egg quality and, therefore, leads to disease or death of the embryo. The result is a higher embryonic mortality or lower DOC quality. These, among others, result from the lower transfer of antioxidants and antibodies from the hen, low viability of the chick’s immune cells, and higher bacterial contamination. A lower relative weight of the bursa of Fabricio and the thymus is often found.
Qreshi’s team (29) studied the effects on the progeny of broiler breeders consuming feed highly contaminated with AFB1, finding suppression in antibody production and macrophage function in chicks after ten days. Similar results were found by other researchers (36, 37) evaluating the effects of AF and OTA as single and combined contamination. When both mycotoxins are present in the feed, the effect on hatchability and DOC quality are synergistic.
Due to mycotoxin contamination, the reproduction and immune response are impaired, resulting in decreased DOC production and increased early chick mortality, as they are more susceptible to bacterial and viral infections.
Mycotoxins impair table egg production and quality
Studies (22-24) have found mycotoxin contamination in commercial table eggs. A meta-analysis of mycotoxins’ concentration based on 11 published papers was completed recently (22): counting with data from 9509 samples, the meta-analysis reveals an overall presence of mycotoxins in 30% of the samples, being Beauvericin in the first place, followed by DON as well as AF and OTA in third and fourth place, respectively. The risk for humans depends on the intake of contaminated foods in terms of amount and frequency (25), and so far, it has not been estimated in most parts of the world.
Natural contamination in laying hens: a case report
Giancarlo Bozzo’s team (39) reported and published a veterinary case regarding natural mycotoxin contamination in commercial egg production: up to week 47 of age, production parameters were on top of the genetic standards. However, a drop in egg production started at around week 47, and at week 50, egg production was only 68% (figure 1).
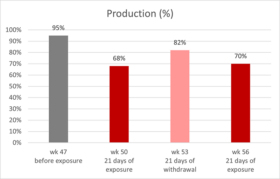
Figure 1: production of laying hens fed naturally contaminated feed with AFB1 and OTA
Samples were collected at week 56, and AFB1 and OTA were detected in feed and the kidneys and livers of the hens consuming it (table 2). While the levels in the feed were not considered high risk, evidence from necropsy and histopathology suggested either a higher or a prolonged exposure; a synergistic effect of both mycotoxins on hen’s health and productivity can be inferred.
Table 2: mycotoxin analysis results for feed and organs
| HPLC analysis results in samples of: | ||||
| toxin | Feed 1 (n=5) |
Feed 2 (n=5) |
Kidney
(n=10) |
Liver
(n=10) |
| OTA | 1.1 ± 0.1 ppb | 31 ± 3 ppb | 47 ± 3 ppb | 24 ± 2 ppb |
| AFB1 | ND | 5.6 ± 0.3 ppb | 1.4 ± 0.3 ppb | 3.6 ± 0.4 ppb |
The liver and kidneys were enlarged and showed signs of damage. Furthermore, urate crystals in the peritoneum and the abdominal air sac were observed, indicating renal failure. This limited the excretion of both toxins in the urine, increasing their half-life in the organism and enhancing the effects in target organs, contributing to the synergistic effect observed.
After using mycotoxin-free certified linseed, the problem receded. Though this is the best option to keep animals healthy and productive, it may not be practical in the long term due to the ubiquitous nature of the toxins and the cost and availability constraints of feed raw materials. Moreover, the mycotoxin levels present in the feed were relatively low and fell under recommended guidelines. For these reasons, in-feed toxin mitigation solutions must also be considered to reduce exposure for production animals.
In-feed intervention mitigates the effects of intermittent exposure to multiple mycotoxins
EW Nutrition conducted a study with Hy-Line W-36 layer-breeders intercalating three 10-day cycles of feed with 100ppb AFB1 + 100ppb OTA, with two 21-day cycles of non-challenged feed. An in-feed intervention (Solis Max 2.0, displayed as IFI) containing bentonite, yeast cell wall components, and a mixture of phytogenic components mitigated all effects.
Table 3: experimental groups and mycotoxin challenge
| Treatment | Group | 100 ppb AFB1+ 100 ppb OTA | IFI (2 kg/ton) |
| T-1 | Control (C) | ||
| T-2 | C+IFI | X | |
| T-3 | Challenge (Ch) | X | |
| T-4 | Ch+IFI | X | X |
Trial design:
A total of 576 hens (18 replicates per diet, 8 hens each) and 58 roosters were randomly assigned to four diets at 28 weeks of age, as shown in Table 3. The 72-day experimental period included alternating 10-day challenge and 21-day non-challenge intervals (Figure 2). During the challenge intervals, the breeders in T-3 and T-4 were fed the mycotoxin-contaminated feed with and without the IFI.
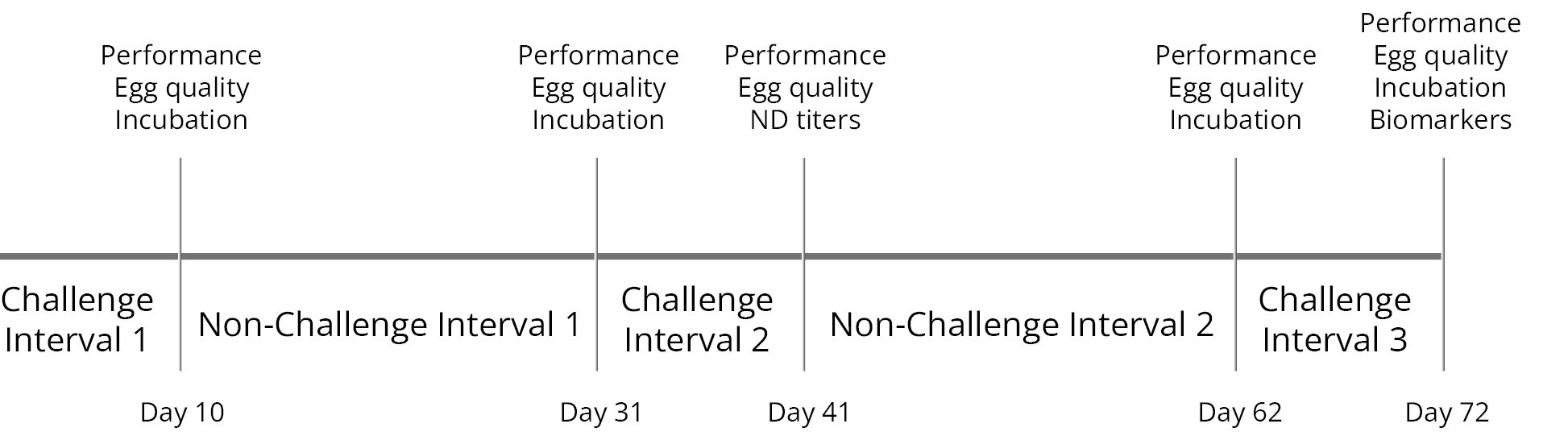 Figure 2: trial timeline showing challenge and non-challenge intervals and days of data collection and sampling.
Figure 2: trial timeline showing challenge and non-challenge intervals and days of data collection and sampling.
Mitigated effects on egg production and egg quality
The challenge decreased overall egg production (Figure 3), egg mass, and shell thickness (Table 4). The first challenge interval did not affect production, but days later, from the first non-challenge period, all parameters were lower for the challenged group.
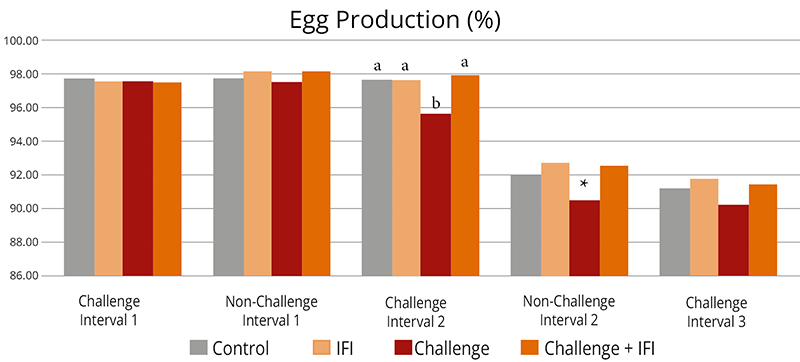 Different letters indicate significant differences (p<0.05). Statistical tendencies (p<0.1) are indicated by (*).
Different letters indicate significant differences (p<0.05). Statistical tendencies (p<0.1) are indicated by (*).
Figure 3: Egg production of hens intermittently challenged with AFB1 and OTA, with and without in-feed Solis Max
The adverse effects on productivity and egg quality started after the first challenged feed was withdrawn and persisted through the following intervals until the end of the experiment. Similar effects in chronic mycotoxin challenges have been previously found (37, 39).
Table 4: Average egg quality parameters of hens intermittently challenged with AFB1+OTA, with and without an in-feed intervention (IFI)
| Group | Eggshell strength (N) | Eggshell thickness (mm) | Haugh Units |
| Control | 21,02a | 0,3661ab | 70,88 |
| IFI | 21,16a | 0,3702a | 71,68 |
| Challenge | 20,05b | 0,3630b | 70,07* |
| Ch+IFI | 21,06a | 0,3698a | 71,06 |
Different letters indicate significant differences (p<0.05). Statistical tendencies (p<0.1) are indicated by (*).
Mitigated effects on the progeny in incubation trials
Three incubation trials were performed: after the first challenge and non-challenge interval and at the end of the trial period after the third challenge interval. A significant decrease in fertility and hatchability was observed for the challenged group in all incubation trials. As mycotoxins affect egg quality (22-24) and can be transferred to the eggs (10, 14, 27), the effects were also shown in the case of hatchability and offspring performance. Fertility was affected from the first challenge interval onwards, continuing to be low for the challenge group until the end of the trial. However, the hatchability of fertile eggs dropped after the withdrawal of the contaminated feed and showed the lowest value during the third challenge interval.
The in-feed supplementation of Solis Max 2.0 (IFI) resulted in the consistent recovery of egg production and egg quality throughout the whole experimental period, achieving the same levels of productivity as the non-challenged control.

Figure 4: Hatchery parameters of eggs from breeders intermittently challenged with AFB1 and OTA, with and without an in-feed intervention (IFI).
Results in hatch of fertile can be related to egg quality, as the thickness of the eggshell influences the egg’s moisture loss and exchange with the environment during the incubation period. Thinner eggshells lead to higher embryo mortality (31, 32). The group having the challenge with Solis Max showed the same performance as the non-challenged control regarding hatchery performance.
Day-old chick weight was not affected. However, weight gain and mortality after ten days were hindered for the chicks from breeders taking the mycotoxin-contaminated feed (Table 5).
Table 5: Average day- and 10-day-old chick parameters from hens intermittently challenged with AFB1+OTA, with and without an in-feed intervention (IFI)
| Parameter | Control | Challenge | Ch + IFI |
| DOC body weight (g) | 36,67 | 36,24 | 36,80 |
| 10-day body weight (g) | 76,30a | 75,94b | 79,50a |
| 10-day mortality (%) | 0,94 | 1,26 | 0,97 |
Letters indicate significant differences (p<0.05). Statistical tendencies (p<0.1) indicated by (*)
At the end of the experiment, oxidative stress biomarkers were measured in the blood serum of 15 hens per treatment, showing significantly lower GPx, and SOD (figure 5) in the challenged group, which indicates a depletion of the mechanisms to fight oxidative stress (40), the hens taking the in-feed product did not show this depletion.
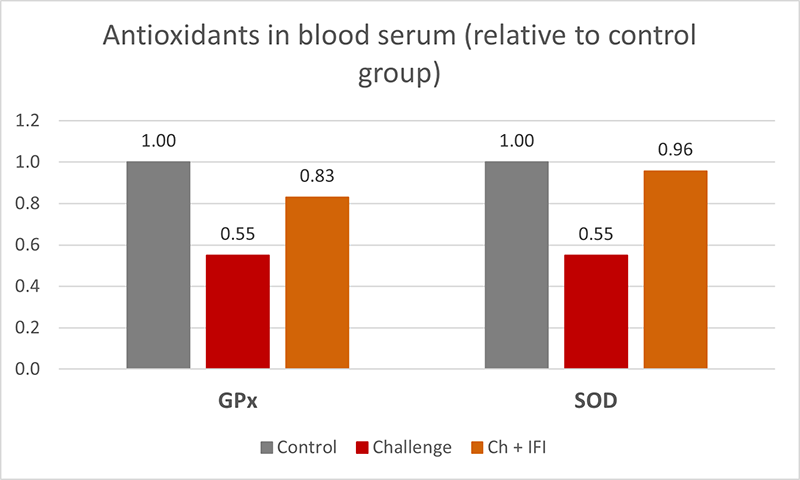 Figure 5: Antioxidants in blood serum, glutathione peroxidase (GPx), and superoxide dismutase (SOD) from breeders intermittently challenged with AFB1 and OTA, with and without an in-feed intervention (IFI).
Figure 5: Antioxidants in blood serum, glutathione peroxidase (GPx), and superoxide dismutase (SOD) from breeders intermittently challenged with AFB1 and OTA, with and without an in-feed intervention (IFI).
Intermittent exposure to AFB1 and OTA negatively affected layer breeder productivity, egg quality, and hatchability and promoted oxidative stress in the birds. Intermittent mycotoxin challenges may affect animals even after the contamination is withdrawn. In-feed interventions showed effectiveness in mitigating these effects.
Climate changes bring new mycotoxin challenges – the right in-feed solutions can help
Today’s mycotoxin scenario shows increased frequency, quantity, and variety. Mainly long-living animals, such as laying hens and breeders, can be at more risk. Additionally, the contamination can be carried over to the eggs, potentially risking human health in the case of table eggs and hatchery performance and DOC quality in the case of breeders.
From case reports, we learn the consequences of real challenges and struggles in commercial production; from scientific trials based on possible commercial situations, we realize the advantages of interventions designed to tackle those challenges.
References
- Kos, Jovana, Mislav Anić, Bojana Radić, Manuela Zadravec, Elizabet Janić Hajnal, and Jelka Pleadin. “Climate Change—a Global Threat Resulting in Increasing Mycotoxin Occurrence.” Foods 12, no. 14 (July 14, 2023): 2704. https://doi.org/10.3390/foods12142704.
- Zingales, Veronica, Mercedes Taroncher, Piera Anna Martino, María-José Ruiz, and Francesca Caloni. “Climate Change and Effects on Molds and Mycotoxins.” Toxins 14, no. 7 (June 30, 2022): 445. https://doi.org/10.3390/toxins14070445.
- Loi, Martina, Antonio F. Logrieco, Tünde Pusztahelyi, Éva Leiter, László Hornok, and István Pócsi. “Advanced Mycotoxin Control and Decontamination Techniques in View of an Increased Aflatoxin Risk in Europe Due to Climate Change.” Frontiers in Microbiology 13 (January 10, 2023). https://doi.org/10.3389/fmicb.2022.1085891.
- Medina, Ángel, Jesús M González-Jartín, and María J Sainz. “Impact of Global Warming on Mycotoxins.” Current Opinion in Food Science 18 (December 2017): 76–81. https://doi.org/10.1016/j.cofs.2017.11.009.
- Grenier, Bertrand, and Todd Applegate. “Modulation of Intestinal Functions Following Mycotoxin Ingestion: Meta-Analysis of Published Experiments in Animals.” Toxins 5, no. 2 (February 21, 2013): 396–430. https://doi.org/10.3390/toxins5020396.
- Filazi, Ayhan, Begum Yurdakok-Dikmen, Ozgur Kuzukiran, and Ufuk Tansel Sireli. “Mycotoxins in Poultry.” Poultry Science, February 15, 2017. https://doi.org/10.5772/66302.
- Amirkhizi, Behzad, Seyed Rafie Arefhosseini, Masoud Ansarin, and Mahboob Nemati. “Aflatoxin B1in Eggs and Chicken Livers by Dispersive Liquid–Liquid Microextraction and HPLC.” Food Additives & Contaminants: Part B, August 27, 2015, 1–5. https://doi.org/10.1080/19393210.2015.1067649.
- Emmanuel K, Tangni, Van Pamel Els, Huybrechts Bart, Delezie Evelyne, Van Hoeck Els, and Daeseleire Els. “Carry-over of Some Fusarium Mycotoxins in Tissues and Eggs of Chickens Fed Experimentally Mycotoxin-Contaminated Diets.” Food and Chemical Toxicology 145 (November 2020): 111715. https://doi.org/10.1016/j.fct.2020.111715.
- Ebrahem, Mohammad, Susanne Kersten, Hana Valenta, Gerhard Breves, and Sven Dänicke. “Residues of Deoxynivalenol (Don) and Its Metabolite de-Epoxy-Don in Eggs, Plasma and Bile of Laying Hens of Different Genetic Backgrounds.” Archives of Animal Nutrition 68, no. 5 (August 20, 2014): 412–22. https://doi.org/10.1080/1745039x.2014.949029.
- Salwa, A. Aly, and W. Anwer. “Effect of Naturally Contaminated Feed with Aflatoxins on Performance of Laying Hens and the Carryover of Aflatoxin B1 Residues in Table Eggs.” Pakistan Journal of Nutrition 8, no. 2 (January 15, 2009): 181–86. https://doi.org/10.3923/pjn.2009.181.186.
- Devreese, Mathias, Gunther Antonissen, Nathan Broekaert, Siegrid De Baere, Lynn Vanhaecke, Patrick De Backer, and Siska Croubels. “Comparative Toxicokinetics, Absolute Oral Bioavailability, and Biotransformation of Zearalenone in Different Poultry Species.” Journal of Agricultural and Food Chemistry 63, no. 20 (May 19, 2015): 5092–98. https://doi.org/10.1021/acs.jafc.5b01608.
- Galtier, P. “Biotransformation and Fate of Mycotoxins.” Toxin Reviews 18, no. 3 (August 1, 1999): 295–312. https://doi.org/10.3109/15569549909162648.
- Galtier, P., M. Alvinerie, and J.L. Charpenteau. “The Pharmacokinetic Profiles of Ochratoxin A in Pigs, Rabbits and Chickens.” Food and Cosmetics Toxicology 19 (January 1981): 735–38. https://doi.org/10.1016/0015-6264(81)90528-9.
- Hassan, Zahoor Ul, Muhammad Z Khan, Ahrar Khan, Ijaz Javed, and Zahid Hussain. “Effects of Individual and Combined Administration of Ochratoxin A and Aflatoxin B1 in Tissues and Eggs of White Leghorn Breeder Hens.” Journal of the Science of Food and Agriculture 92, no. 7 (December 16, 2011): 1540–44. https://doi.org/10.1002/jsfa.4740.
- Li, Shao-Ji, Guangzhi Zhang, Bin Xue, Qiaoling Ding, Lu Han, Jian-chu Huang, Fuhai Wu, Chonggao Li, and Chunmin Yang. “Toxicity and Detoxification of T-2 Toxin in Poultry.” Food and Chemical Toxicology 169 (November 2022): 113392. https://doi.org/10.1016/j.fct.2022.113392.
- Prelusky, D.B., R.M.G. Hamilton, and H.L. Trenholm. “Transmission of Residues to Eggs Following Long-Term Administration of 14 C-Labelled Deoxynivalenol to Laying Hens.” Poultry Science 68, no. 6 (June 1989): 744–48. https://doi.org/10.3382/ps.0680744.
- Ringot, Diana, Abalo Chango, Yves-Jacques Schneider, and Yvan Larondelle. “Toxicokinetics and Toxicodynamics of Ochratoxin A, an Update.” Chemico-Biological Interactions 159, no. 1 (January 2006): 18–46. https://doi.org/10.1016/j.cbi.2005.10.106.
- Osselaere, Ann, Mathias Devreese, Joline Goossens, Virginie Vandenbroucke, Siegrid De Baere, Patrick De Backer, and Siska Croubels. “Toxicokinetic Study and Absolute Oral Bioavailability of Deoxynivalenol, T-2 Toxin and Zearalenone in Broiler Chickens.” Food and Chemical Toxicology 51 (January 2013): 350–55. https://doi.org/10.1016/j.fct.2012.10.006.
- Sudhakar, BV. “A Study on Experimentally Induced Aflatoxicosis on the Carryover of Aflatoxin B1 into Eggs and Liver Tissue of White Leghorn Hens.” The Pharma Innovation Journal 11, no. 2S (2022): 213–17.
- Yiannikouris, Alexandros, and Jean-Pierre Jouany. “Mycotoxins in Feeds and Their Fate in Animals: A Review.” Animal Research 51, no. 2 (March 2002): 81–99. https://doi.org/10.1051/animres:2002012.
- Bouhet, Sandrine, and Isabelle P. Oswald. “The Intestine as a Possible Target for Fumonisin Toxicity.” Molecular Nutrition & Food Research 51, no. 8 (August 2007): 925–31. https://doi.org/10.1002/mnfr.200600266.
- Fakhri, Yadolah, Mansour Sarafraz, Amene Nematollahi, Vahid Ranaei, Moussa Soleimani-Ahmadi, Van Nam Thai, and Amin Mousavi Khaneghah. “A Global Systematic Review and Meta-Analysis of Concentration and Prevalence of Mycotoxins in Birds’ Egg.” Environmental Science and Pollution Research 28, no. 42 (September 9, 2021): 59542–50. https://doi.org/10.1007/s11356-021-16136-y.
- Osaili, Tareq M., Akram R. Al-Abboodi, Mofleh AL. Awawdeh, and Samah Aref Jbour. “Assessment of Mycotoxins (Deoxynivalenol, Zearalenone, Aflatoxin B1 and Fumonisin B1) in Hen’s Eggs in Jordan.” Heliyon 8, no. 10 (October 2022). https://doi.org/10.1016/j.heliyon.2022.e11017.
- Wang, Lan, Qiaoyan Zhang, Zheng Yan, Yanglan Tan, Runyue Zhu, Dianzhen Yu, Hua Yang, and Aibo Wu. “Occurrence and Quantitative Risk Assessment of Twelve Mycotoxins in Eggs and Chicken Tissues in China.” Toxins 10, no. 11 (November 16, 2018): 477. https://doi.org/10.3390/toxins10110477.
- Tolosa, J., Y. Rodríguez-Carrasco, M.J. Ruiz, and P. Vila-Donat. “Multi-Mycotoxin Occurrence in Feed, Metabolism and Carry-over to Animal-Derived Food Products: A Review.” Food and Chemical Toxicology 158 (December 2021): 112661. https://doi.org/10.1016/j.fct.2021.112661.
- Völkel, Inger, Eva Schröer-Merker, and Claus-Peter Czerny. “The Carry-over of Mycotoxins in Products of Animal Origin with Special Regard to Its Implications for the European Food Safety Legislation.” Food and Nutrition Sciences 02, no. 08 (2011): 852–67. https://doi.org/10.4236/fns.2011.28117.
- Yuan, Tao, Junyi Li, Yanan Wang, Meiling Li, Ao Yang, Chenxi Ren, Desheng Qi, and Niya Zhang. “Effects of Zearalenone on Production Performance, Egg Quality, Ovarian Function and Gut Microbiota of Laying Hens.” Toxins 14, no. 10 (September 21, 2022): 653. https://doi.org/10.3390/toxins14100653.
- Song, Bin, Teng Ma, Damien P. Prévéraud, Keying Zhang, Jianping Wang, Xuemei Ding, Qiufeng Zeng, et al. “Research Note: Effects of Feeding Corn Naturally Contaminated with Aflatoxin B1, Deoxynivalenol, and Zearalenone on Reproductive Performance of Broiler Breeders and Growth Performance of Their Progeny Chicks.” Poultry Science 102, no. 11 (November 2023): 103024. https://doi.org/10.1016/j.psj.2023.103024.
- Qureshi, MA, J Brake, PB Hamilton, WM Hagler, and S Nesheim. “Dietary Exposure of Broiler Breeders to Aflatoxin Results in Immune Dysfunction in Progeny Chicks.” Poultry Science 77, no. 6 (June 1998): 812–19. https://doi.org/10.1093/ps/77.6.812.
- Ul-Hassan, Zahoor, Muhammad Zargham Khan, Ahrar Khan, and Ijaz Javed. “Immunological Status of the Progeny of Breeder Hens Kept on Ochratoxin a (OTA)- and Aflatoxin B1(Afb1)-Contaminated Feeds.” Journal of Immunotoxicology 9, no. 4 (April 24, 2012): 381–91. https://doi.org/10.3109/1547691x.2012.675365.
- Devegowda, G., and D. Ravikiran. “Mycotoxins and Eggshell Quality: Cracking the Problem.” World Mycotoxin Journal 1, no. 2 (May 1, 2008): 203–8. https://doi.org/10.3920/wmj2008.1037.
- Onagbesan, O., V. Bruggeman, L. De Smit, M. Debonne, A. Witters, K. Tona, N. Everaert, and E. Decuypere. “Gas Exchange during Storage and Incubation of Avian Eggs: Effects on Embryogenesis, Hatchability, Chick Quality and Post-Hatch Growth.” World’s Poultry Science Journal 63, no. 4 (December 1, 2007): 557–73. https://doi.org/10.1017/s0043933907001614.
- Ebrahem, Mohammad, Susanne Kersten, Hana Valenta, Gerhard Breves, Andreas Beineke, Kathrin Hermeyer, and Sven Dänicke. “Effects of Feeding Deoxynivalenol (Don)-Contaminated Wheat to Laying Hens and Roosters of Different Genetic Background on the Reproductive Performance and Health of the Newly Hatched Chicks.” Mycotoxin Research 30, no. 3 (April 11, 2014): 131–40. https://doi.org/10.1007/s12550-014-0197-z.
- Yegani, M., T.K. Smith, S. Leeson, and H.J. Boermans. “Effects of Feeding Grains Naturally Contaminated with Fusarium Mycotoxins on Performance and Metabolism of Broiler Breeders.” Poultry Science 85, no. 9 (September 2006): 1541–49. https://doi.org/10.1093/ps/85.9.1541.
- Calini, F, and F Sirri. “Breeder Nutrition and Offspring Performance.” Revista Brasileira de Ciência Avícola 9, no. 2 (June 2007): 77–83. https://doi.org/10.1590/s1516-635×2007000200001.
- Hassan, ZU, MZ Khan, A Khan, I Javed, U Sadique, and A Khatoon. “Ochratoxicosis in White Leghorn Breeder Hens: Production and Breeding Performance.” Vet. J. 32, no. 4 (2012): 557–61.
- Verma, J., T. S. Johri, and B. K. Swain. “Effect of Varying Levels of Aflatoxin, Ochratoxin and Their Combinations on the Performance and Egg Quality Characteristics in Laying Hens.” Asian-Australasian Journal of Animal Sciences 16, no. 7 (January 1, 2003): 1015–19. https://doi.org/10.5713/ajas.2003.1015.
- Johnson-Dahl, M.L., M.J. Zuidhof, and D.R. Korver. “The Effect of Maternal Canthaxanthin Supplementation and Hen Age on Breeder Performance, Early Chick Traits, and Indices of Innate Immune Function.” Poultry Science 96, no. 3 (March 2017): 634–46. https://doi.org/10.3382/ps/pew293.
- Bozzo, Giancarlo, Nicola Pugliese, Rossella Samarelli, Antonella Schiavone, Michela Maria Dimuccio, Elena Circella, Elisabetta Bonerba, Edmondo Ceci, and Antonio Camarda. “Ochratoxin A and Aflatoxin B1 Detection in Laying Hens for Omega 3-Enriched Eggs Production.” Agriculture 13, no. 1 (January 5, 2023): 138. https://doi.org/10.3390/agriculture13010138.
- Surai, Peter F., Ivan I. Kochish, Vladimir I. Fisinin, and Michael T. Kidd. “Antioxidant Defence Systems and Oxidative Stress in Poultry Biology: An Update.” Antioxidants 8, no. 7 (July 22, 2019): 235. https://doi.org/10.3390/antiox8070235.















