By Dr. Inge Heinzl, Editor EW Nutrition
Gut health is a critical factor in poultry production, influencing growth performance, feed efficiency, and overall bird health. A well-functioning digestive system ensures optimal nutrient absorption and ultimately contributes to economic sustainability in poultry farming.
However, another essential function of the gut is its significant role in immune defense, as evidenced by the fact that 80% of all active immune cells are in the gut. It is essential for the organism to keep a sensitive balance by eliminating invading pathogens while maintaining self-tolerance to avoid autoimmunity. Being 1.5 to 2.3 m long and with a big contact area to the external environment, the gut is the first line of defense when pathogens have orally entered the organism. For this purpose, the intestine has several specialized cells and a plethora of diverse microorganisms – the microbiome.
A balanced gut environment, therefore, enhances resistance to diseases, helps prevent infections, and reduces the need for antibiotics.
Which tools are available in the gut to counteract pathogenic attacks?
The gut wall, per se, has several fixed tools to fight pathogenic offenses, such as the mucus layers and the epithelium with highly specialized cells. Figure 1 shows in detail the different parts of the gut immune system.
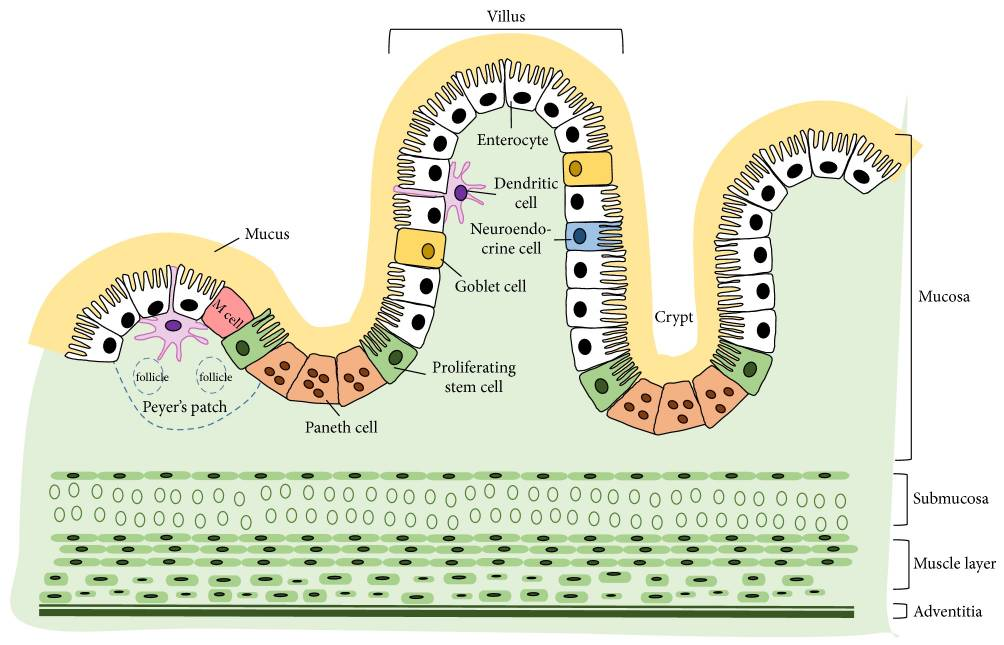
1. Mucus layers
The mucus layers form the first host-derived line of defense. They help trap invasive bacteria and facilitate their removal via luminal flow. The protective properties may depend on whether the mucin is neutral or acidic, sialylated or sulfated (Broom and Kogut, 2018). The glycoprotein mucins forming the mucus layer (mainly MUC2 in the small and large intestine and MUC5ac in the proventriculus) are produced by the goblet cells, part of the intestinal epithelium just beneath.
2. Intestinal epithelium
The one-layered intestinal epithelium represents a physical barrier and consists of normal enterocytes, as well as specialized cells. All the cells are closely linked by tight junctions, consisting of claudin, occludin, and junctional adhesion molecules (JAM).
The following diverse specialized cells protect the organism from pathogenic attacks:
2.1 Proliferating stem cells
These cells are ready to replace damaged epithelial cells in the case of inflammation.
2.2 Paneth cells
Paneth cells are situated at the bottom of the Lieberkühn crypts, neighboring the stem cells in the jejunum and the ileum. Paneth cells have different tasks:
In normal conditions, they maintain homeostasis by regulating the microbiome’s composition via the secretion of antimicrobial peptides, which are accumulated in apically oriented secretory granules, performing phagocytosis and efferocytosis. Additionally, the Paneth cells provide niche factors for the intestinal stem cell compartment, absorb heavy metals, and preserve the integrity of the intestinal barrier. If one or more of these functions are impaired, intestinal and systemic inflammations or infections can develop (Wallaeys et al., 2022). The number of Paneth cells and their diameter can be enhanced via feeding. Agarwal et al. (2022) noticed a significant increase in the number and diameter of Paneth cells after feeding quinoa soluble fiber and/or quercetin 3-glucoside.
2.3 M cells
M cells (M coming from microfold and indicating the structure) are specialized epithelial cells localized along the antimesenteric border in the epithelium of the ileum. They are crucial for the immune system and an essential part of the gut-associated lymphoid tissue (GALT), a sub-system of the mucosa-associated lymphoid tissue (MALT).
M cells play an important role in the function of the immune system. They act as a transport system for antigens. They sample antigens (macromolecules, bacteria, viruses, small parasites) via the apical membrane. After the phagocytosis of the foreign organism/substance, the antigen gets through the cell and is consigned to cells of the adaptive immune system (e.g., the B-cells) at the basal side. The exact transport and the handover to the cells of the adaptive immune system are still unclear. It is also not clarified whether the antigens are processed inside the cells.
2.4 Dendritic cells
Dendritic cells are a kind of leucocyte derived from the bone marrow. Immature dendritic cells have a star-like shape. They are specialized to identify, uptake, transport, process, and present antigens to other immune system cells on their surface. To identify and uptake harmful substances/microbes, they carry receptors on their surface that recognize the attributes often occurring in pathogenic viruses, bacteria, and fungi. After contact with the antigen, the cell moves to secondary lymphoid tissue, and in the intestine, this is predominantly Mucosa-Associated Lymphoid Tissue (MALT). Arriving as mature and not phagocytizing dendritic cells, they present the antigens of the pathogens to the T-lymphocytes. For this purpose, they use cell surface proteins (MHC proteins). This presentation, together with co-stimulators and cytokines, activates naïve T-lymphocytes to develop into the relevant T-cell (fighting viruses, bacteria…) and proliferate, leading to the clearance of the pathogen.
On the other hand, dendritic cells can also suppress an immune reaction if the “suspicious subjects” are harmless or belong to the organism. Dendritic cells are the most potent antigen-presenting cells of the immune system.
2.5 Goblet cells
Goblet cells originate from pluripotent stem cells and are located between the enterocytes in the inner mucus layer of the intestine. Goblet cells develop and mature rapidly after hatching due to external stimuli such as environmental and dietary factors, but also intestinal microbiota (Duangnumsawang et al., 2021). They derive their name from their goblet-like appearance. The basal site is thin, but the cell gets thicker toward the apical side. In the thicker cell organisms, vesicles with mucins are stored and explosively released to the surface by exocytosis.
The mucins (MUC2) are viscous, slime-forming substances consisting of a protein string bound to many sugar chains. Due to their oligosaccharide chain structure, they offer adhesion binding sites for intestinal commensal bacteria and enhance probiotic colonization (Liu et al, 2020). They have a high water-binding capacity, which is responsible for their slimy and protective characteristics. In the case of inflammation, mucin production can increase strongly.
By providing bicarbonate for proper mucin unfolding in the small intestine, goblet cells help maintain homeostasis and the intestinal barrier function. Furthermore, goblet cells can form goblet cell-associated passages (GAPs) and deliver luminal substances to the antigen-presenting cells in the underlying lamina propria that can start an adaptive immune response (Knoop and Newberry, 2018).
As with Paneth cells, the number of goblet cells also increases by feeding quinoa soluble fibers.
2.6 Neuroendocrine cells
Enterochromaffin cells are neuroendocrine cells found in the epithelium of the whole digestive tract, mainly in the small intestine, the colon, and the ceca. They belong to the enteric endocrine system, are part of the diffuse neuroendocrine system, and produce 95% of the serotonin in the organism. Enterochromaffin cells act as chemo- and mechanosensors. They react to free fatty acids, amino acids, and other chemicals as well as physical forces occurring during peristaltic activity in the gut, thus modulating the secretion of water and electrolytes as well as gut motility and visceral sensation of pain (Linan-Rico et al., 2016; Diwakarla et al., 2018).
Serotonin, on its side, has been shown to affect the composition of the gut microbiota (Kwon et al., 2019) and to modulate bacterial physiology (Knecht et al., 2016). Gut-derived serotonin is responsible for immune responses (Baganz and Blakely, 2012) but also for the regulation of other functions such as bone development (Chabbi-Achengli et al., 2012), gut motility, and platelet aggregation (Berger et al., 2009). A deficient serotonergic system can cause psychopathological behaviors such as feather pecking.
3. Last but not least – the microbiome
The poultry gut microbiome consists of bacteria, fungi, protozoa, and viruses. Beneficial microbes, such as Lactobacillus, Bifidobacterium, and Bacteroides, contribute to gut health and immunity.
On the one hand, microbes are involved in digestion and nutrient synthesis. They assist in breaking down fiber, producing short-chain fatty acids, and synthesizing essential vitamins. On the other hand, they contribute to immune defense:
Beneficial bacteria (BB) prevent the colonization of harmful microbes:
The bacteria inhabiting the poultry gut act against pathogens by competing with them for nutrients and binding sites at the intestinal mucosa.
Beneficial bacteria prevent/reduce inflammation and stabilize the intestinal mucosa
Abaidullah et al. (2019) showed in their review how beneficial bacteria influence the immune response to diverse viruses (AIV, IBDV, MDV, NDV).
Bacteria such as Collinsella, Faecalibacterium, Oscillibacter, etc., increase the release of IFN-α, IFN-β, and IL-22. These substances control virus replication and repair mucosal tissue damage. Other bacteria, such as Clostridium XIVa or Firmicutes, provoke T-cells to produce anti-inflammatory cytokines to suppress inflammation. By promoting the antimicrobial peptides such as MUC, TFF, ZO, and tight junction proteins comprised of claudins, occludin, and zona occludens mRNA expression, Bacteroides, Candidatus, SMB53, Parabacteroides, Lactobacillus, Paenibacillus, Enterococcus, and Streptococcus spp. inhibit pathobiont colonization and translocation, and suppress inflammation. Butyrate succinate and lactate, produced by Faecalibacterium and Blautia spp., provide energy and reduce inflammation.
Bacteroides fragilis produce bacterial polysaccharides that communicate with the immune system and influence the transformation of CD4+ (T-helper cells) and Foxp3+ cells (the master transcription factor of regulatory T cells in mammals, but also present in chicken (Burkhardt et al., 2022)).
“Negative” bacteria increase inflammation and enhance viral shedding
Clostridium Cluster XI, Salmonella, and Shigella downregulate the anti-inflammatory and tight junction-stabilizing substances, which would be increased by the beneficial bacteria and increase IFN-γ and IF-17A to cause mucosal inflammation and tissue damage, as well as increased virus replication and fecal shedding. Further bacteria, which enhance mucosal and GIT inflammation, are Desulfovibrionaceae, producing hydrogen sulfides, Vampirovibrio, Clostridium cluster XIVb, and the genus Rumicoccus. They induce the pro-inflammatory cytokines IL-6 and IL-1β. The latter three bacteria also increase viral shedding. Salmonella typhimurium and Campylobacter jejuni also achieve higher viral shedding by decreasing viral-specific IgG and IgA production (Abaidullah et al., 2019)
Factors impairing intestinal immune defense
As the previous paragraph indicates, an imbalance of the intestinal microbiome called dysbiosis makes chickens more prone to diseases such as necrotic enteritis (Stanley et al., 2014). Several factors are disturbing the balance in the microbiome (Heinzl, 2020):
- An abrupt change of feed
- High contents of non-starch polysaccharides increase viscosity, decrease passage rate, lower the digestibility of other nutrients, and serve as nutrients for, e.g., Clostridium perfringens
- High protein levels can also serve as a substrate for pathogens and cause a shift in the balance of the intestinal flora
- Finely ground feed does not stimulate the gizzard muscles to do their work. pH increases, transit time decreases, and pathogenic microbes such as Salmonella, Campylobacter, and Clostridia proliferate.
- Stress (heat or cold stress, re-assembling of groups, high stocking densities)
- Mycotoxins
However, besides all these factors causing an overgrowth of commensal bacteria such as E. coli, ingested pathogens such as Marek’s or Newcastle Disease viruses can also cause this imbalance.
Immune defense in the gut – an interplay of different tools that must be protected
The first line of defense, the intestine, comprises different tools working together to fight pathogens and harmful substances. Besides the mucus layers and the specialized cells, the intestinal microbiome plays an essential role in immune defense by competing with pathogens for nutrients and binding sites, enhancing the secretion of anti-inflammatory substances, and stimulating the production of interferons, which fight the pathogens. However, several factors can impact the balance of the microbiome and cause dysbiosis. The best protection of this sensitive equilibrium can support the organism in defending against diseases and maintaining immunity and performance. Understanding the interplay between microbiota, immune function, and nutrition allows for effective strategies to enhance poultry health while reducing reliance on antibiotics. Future research will continue to provide insights into optimizing gut-immune interactions in poultry production.
References
Abaidullah, Muhammad, Shuwei Peng, Muhammad Kamran, Xu Song, and Zhongqiong Yin. “Current Findings on Gut Microbiota Mediated Immune Modulation against Viral Diseases in Chicken.” Viruses 11, no. 8 (July 25, 2019): 681. https://doi.org/10.3390/v11080681.
Baganz, Nicole L., and Randy D. Blakely. “A Dialogue between the Immune System and Brain, Spoken in the Language of Serotonin.” ACS Chemical Neuroscience 4, no. 1 (December 24, 2012): 48–63. https://doi.org/10.1021/cn300186b.
Berger, Miles, John A. Gray, and Bryan L. Roth. “The Expanded Biology of Serotonin.” Annual Review of Medicine 60, no. 1 (February 1, 2009): 355–66. https://doi.org/10.1146/annurev.med.60.042307.110802.
Broom, Leon J., and Michael H. Kogut. “The Role of the Gut Microbiome in Shaping the Immune System of Chickens.” Veterinary Immunology and Immunopathology 204 (October 2018): 44–51. https://doi.org/10.1016/j.vetimm.2018.10.002.
Burkhardt, Nina B, Daniel Elleder, Benjamin Schusser, Veronika Krchlíková, Thomas W Göbel, Sonja Härtle, and Bernd Kaspers. “The Discovery of Chicken Foxp3 Demands Redefinition of Avian Regulatory T Cells.” The Journal of Immunology 208, no. 5 (March 1, 2022): 1128–38. https://doi.org/10.4049/jimmunol.2000301.
Chabbi-Achengli, Yasmine, Amélie E. Coudert, Jacques Callebert, Valérie Geoffroy, Francine Côté, Corinne Collet, and Marie-Christine de Vernejoul. “Decreased Osteoclastogenesis in Serotonin-Deficient Mice.” Proceedings of the National Academy of Sciences 109, no. 7 (January 30, 2012): 2567–72. https://doi.org/10.1073/pnas.1117792109.
Clarke, G, S Grenham, P Scully, P Fitzgerald, R D Moloney, F Shanahan, T G Dinan, and J F Cryan. “The Microbiome-Gut-Brain Axis during Early Life Regulates the Hippocampal Serotonergic System in a Sex-Dependent Manner.” Molecular Psychiatry 18, no. 6 (June 2013): 666–73. https://doi.org/10.1038/mp.2012.77.
Diwakarla, S., L. J. Fothergill, J. Fakhry, B. Callaghan, and J. B. Furness. “Heterogeneity of Enterochromaffin Cells within the Gastrointestinal Tract.” Neurogastroenterology & Motility 29, no. 6 (May 9, 2017). https://doi.org/10.1111/nmo.13101.
Duangnumsawang, Yada, Jürgen Zentek, and Farshad Goodarzi Boroojeni. “Development and Functional Properties of Intestinal Mucus Layer in Poultry.” Frontiers in Immunology 12 (October 4, 2021). https://doi.org/10.3389/fimmu.2021.745849.
Heinzl, Inge. “Necrotic Enteritis: The Complete Overview.” EW Nutrition, August 8, 2023. https://ew-nutrition.com/necrotic-enteritis-complete-overview/.
Knecht, Leslie D., Gregory O’Connor, Rahul Mittal, Xue Z. Liu, Pirouz Daftarian, Sapna K. Deo, and Sylvia Daunert. “Serotonin Activates Bacterial Quorum Sensing and Enhances the Virulence of Pseudomonas Aeruginosa in the Host.” EBioMedicine 9 (July 2016): 161–69. https://doi.org/10.1016/j.ebiom.2016.05.037.
Kong, Shanshan, Yanhui H. Zhang, and Weiqiang Zhang. “Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids.” BioMed Research International 2018 (2018): 1–10. https://doi.org/10.1155/2018/2819154.
Kwon, Yun Han, Huaqing Wang, Emmanuel Denou, Jean-Eric Ghia, Laura Rossi, Michelle E. Fontes, Steve P. Bernier, et al. “Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis.” Cellular and Molecular Gastroenterology and Hepatology 7, no. 4 (2019): 709–28. https://doi.org/10.1016/j.jcmgh.2019.01.004.
Linan-Rico, Andromeda, Fernando Ochoa-Cortes, Arthur Beyder, Suren Soghomonyan, Alix Zuleta-Alarcon, Vincenzo Coppola, and Fievos L. Christofi. “Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation.” Frontiers in Neuroscience 10 (December 19, 2016). https://doi.org/10.3389/fnins.2016.00564.
Liu, Yang, Xinjie Yu, Jianxin Zhao, Hao Zhang, Qixiao Zhai, and Wei Chen. “The Role of MUC2 Mucin in Intestinal Homeostasis and the Impact of Dietary Components on MUC2 Expression.” International Journal of Biological Macromolecules 164 (December 2020): 884–91. https://doi.org/10.1016/j.ijbiomac.2020.07.191.
Lyte, Mark. “Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior.” PLoS Pathogens 9, no. 11 (November 14, 2013). https://doi.org/10.1371/journal.ppat.1003726.
Marcobal, A., P. C. Kashyap, T. A. Nelson, P. A. Aronov, M. S. Donia, A. Spormann, M. A. Fischbach, and J. L. Sonnenburg. “A Metabolomic View of How the Human Gut Microbiota Impacts the Host Metabolome Using Humanized and Gnotobiotic Mice.” The ISME Journal 7, no. 10 (June 6, 2013): 1933–43. https://doi.org/10.1038/ismej.2013.89.
Stanley, Dragana, Shu-Biao Wu, Nicholas Rodgers, Robert A. Swick, and Robert J. Moore. “Differential Responses of Cecal Microbiota to Fishmeal, Eimeria and Clostridium Perfringens in a Necrotic Enteritis Challenge Model in Chickens.” PLoS ONE 9, no. 8 (August 28, 2014). https://doi.org/10.1371/journal.pone.0104739.
Wallaeys, Charlotte, Natalia Garcia‐Gonzalez, and Claude Libert. “Paneth Cells as the Cornerstones of Intestinal and Organismal Health: A Primer.” EMBO Molecular Medicine 15, no. 2 (December 27, 2022). https://doi.org/10.15252/emmm.202216427.
Yano, Jessica M., Kristie Yu, Gregory P. Donaldson, Gauri G. Shastri, Phoebe Ann, Liang Ma, Cathryn R. Nagler, Rustem F. Ismagilov, Sarkis K. Mazmanian, and Elaine Y. Hsiao. “Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis.” Cell 163, no. 1 (September 2015): 258. https://doi.org/10.1016/j.cell.2015.09.017.