By Dr. Inge Heinzl, Editor, EW Nutrition
Salmonellosis is third among foodborne diseases leading to death (Ferrari, 2019). More than 91,000 human cases of Salmonellosis are reported by the EU each year, generating overall costs of up to €3 billion a year (EFSA, 2023), 10-20% of which are attributed to pork consumption (Soumet, 2022). The annual costs arising from the resulting human health losses in 2010 were about €90 million (FCC Consortium, 2010). Take the example of Ireland, where a high prevalence of Salmonella in lymph nodes still shows a severe issue pre-slaughter and a big challenge for slaughterhouses to stick to the process hygiene requirements (Deane, 2022).
Several governments already have monitoring programs in place, and the farms are categorized according to the salmonella contamination of their pigs. In some countries, e.g., Denmark, an economic penalty of 2% of the carcass value must be paid if the farm has level 2 (intermediate seroprevalence) and 4-8% if the level is 3. Other countries, e.g., Germany, the UK, Ireland, or the Netherlands, use quality assurance schemes. The farmers can only sell their carcasses under this label if their farm has a certain level.
Let’s take a quick look at the genus of Salmonella
Salmonellas are rod-shaped gram-negative bacteria of the family of enterobacteria that use flagella for their movement. They were named after the American vet Daniel Elmer Salmon. The genus of Salmonella consists of two species (S. bongori and S. enterica with seven subspecies) with in total more than 2500 serovars (see Figure 1). The effects of the different serovars can range from asymptomatic carriage to severe invasive systemic disease (Gal-Mor, 2014). All Salmonella serovars generally can cause disease in humans; the rosa-marked ones already showed infections.
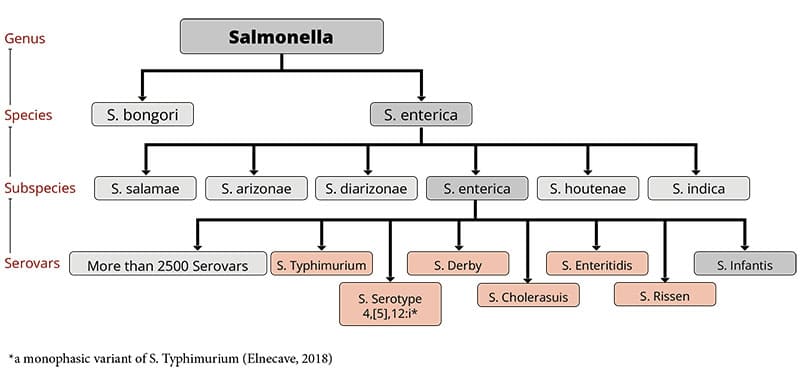 Figure 1: the genus of Salmonella with Salmonella serovars relevant for pigs (according to Bonardi, 2017: Salmonella in the pork production chain and its impact on human health in the European Union)
Figure 1: the genus of Salmonella with Salmonella serovars relevant for pigs (according to Bonardi, 2017: Salmonella in the pork production chain and its impact on human health in the European Union)
Within the group of Salmonella, some serovars can only reside in one or few species, e.g., S. enterica spp. enterica Serovar Dublin (S. Dublin) in bovines (Waldron, 2018) or S. Cholerasuis in pigs (Chiu, 2004). An infection in humans with these pathogens is often invasive and life-threatening (WHO, 2018). On the contrary, serovars like S. Typhimurium and S. Enteritidis are not host-specific and can cause disease in various species.
The serotypes S. Typhi and S. Paratyphi A, B, or C are highly adapted to humans and only for them pathogenic; they are responsible for the occurrence of typhus.
Serovars occurring in pigs and relevant for humans are, for example, S. Typhimurium (Hendriksen, 2004), S. Serotype 4,[5],12:I (Hauser et al., 2010), S. Cholerasuis (Chiu, 2004), S. Derby (Gonzalez-Santamarina, 2021), S. Agona (Brenner Michael, 2006) and S. Rissen (Elbediwi, 2021).
Transmission of Salmonella mostly happens via contaminated food
The way of transmission to humans depends on the serovar:
Human-specific and, therefore, only in humans and higher primates residing serovars S. Typhi and Paratyphi A, B, or C (typhoidal) are excreted via feces or urine. Therefore, any food or water contaminated with the feces or urine of infected people can transmit this disease (Government of South Australia, 2023). Typhoid and paratyphoid Salmonellosis occur endemic in developing countries with the lack of clean water and, therefore, inadequate hygiene (Gal-Mor, 2014).
Serovars which can cause disease in humans and animals (non-typhoidal), can be transmitted by
– animal products such as milk, eggs, meat
– contact with infected persons/animals (pigs, cows, pets, reptiles…) or
– other feces- or urine-contaminated products such as sprouts, vegetables, fruits….
Farm animals take salmonellas from their fellows, contaminated feed or water, rodents, or pests.
Symptoms of Salmonellosis can be severe
In the case of typhoid or paratyphoid Salmonellosis, the onset of illness is gradual. People can suffer from sustained high fever, unwellness, severe headache, and decreased appetite, but also from an enlarged spleen irritating the abdomen and dry cough.
A study conducted in Thailand with children suffering from enteric fever caused by the typhoid serovars S. Typhi and Paratyphi showed a sudden onset of fever and gastrointestinal issues (diarrhea), rose spots, bronchitis, and pneumonia (Thisyakorn et al., 1987)
The non-typhoid Salmonellosis is typically characterized by an acute onset of fever, nausea, abdominal pain with diarrhea, and sometimes vomiting (WHO, 2018). However, 5% of the persons – children with underlying conditions, e.g., babies, or people who have AIDS, malignancies, inflammatory bowel disease, gastrointestinal illness caused by non-typhoid serovars, and hemolytic anemia, or receiving an immunosuppressive therapy can be susceptible to bacteremia. Additionally, serovars like S. Cholerasuis or S. Dublin are apt to develop bacteremia by entering the bloodstream with little or no involvement of the gut (Chiu, 1999). In these cases, consequences can be septic arthritis, pneumonia, peritonitis, cutaneous abscess, mycotic aneurysm, and sometimes death (Chen et al., 2007; Chiu, 2004, Wang et al., 1996).
In pigs, S. Cholerasuis causes high fever, purple discolorations of the skin, and thereinafter diarrhea. The mortality rate in pigs suffering from this type of Salmonellosis is high. Barrows orally challenged with S. Typhimurium showed elevated rectal temperature by 12h, remaining elevated until the end of the study. Feed intake decreased with a peak at 48h after the challenge and remained up to 120h after the challenge. Daily gain reduced during the following two weeks after infection. A higher plasma cortisol level and a lower IGF-I level could also be noticed. All these effects indicate significant changes in the endocrine stress and the somatotropic axis, also without significant alterations in the systemic pro-inflammatory mediators (Balaji et al., 2000)
To protect humans, Salmonella in pork must be restraint
There are three main steps to keep the contamination of pork as low as possible:
- Keeping Salmonella out of the pig farm
- Minimizing spreading if Salmonella is already on the farm
- Minimizing contamination in the slaughterhouse
1. How to keep Salmonella out of the pig farm?
To answer this question, we must look at how the pathogen can be transported to the farm. According to the Code of Practice for the Prevention and Control of Salmonella on Pig Farms (Ministry of Agriculture, Fisheries and Food and the Scottish Executive Rural Affairs Department), there are several possibilities to infiltrate the pathogen into the farm:
- Diseased pigs or pigs which are ill but don’t show any symptoms
- Feeding stuff or bedding contaminated with dung
- Pets, rodents, wild birds, or animals
- Farm personnel or visitors
- Equipment or vehicles
Caution with purchased animals!
To minimize/prevent the entry of Salmonella into the livestock, bought-in animals must come from reputable breeding farms with a salmonella monitoring system in place. As possible carrier animals are more likely to excrete Salmonella when stressed; they should be kept in isolation after purchasing. Additionally, the animals must go through a disinfectant foot bath before entering the farm.
Keep rodents, wild animals, and vermin in check!
Generally, the production site must be kept clean and as unattractive as possible for all these animals. Rests of feed must be removed, and dead animals and afterbirths must be promptly and carefully disposed of. A well-planned baiting and trapping policy should be in place to effectively control rodents.
Only selected people should enter the hog houses
In any case, the number of persons entering the hog house must be kept as low as possible. Farmworkers should be trained in the principles of hygiene. They should wear adequate clothing (waterproof boots and protective overalls) that can be easily cleaned/laundered and disinfected. The clothes/shoes should always be used only at this site. Thorough hand washing and the disinfection of the boots when entering and leaving the pig unit are a must.
If visits are necessary, the visitors should take the same measures as the farm workers. And, of course, they should not have had contact with another pig farm during the last 48 hours.
Keep pens, farm equipment, and vehicles clean!
Farm equipment should not be shared with other farms. If this cannot be avoided, it must be cleaned and disinfected before re-entering the farm. Also, the vehicles for the transport of the animals must be cleaned and disinfected as soon as possible after usage, as contaminated transporters always pose the risk of infection.
Feed should be Salmonella-free!
To get high feed quality, the feed should be purchased from feed mills/sources with a well-functioning bacterial control to guarantee the absence of Salmonella. It is essential that birds, domestic and wild animals cannot enter the feed stores.
It is also advised to keep dry feed dry as possibly contaminating Salmonella can multiply in such humid conditions. Additionally, all feed bins and delivery pipes for dry and wet feed must be consciously cleaned, and the damp feed pipes also disinfected.
The change from pellets to mash could be helpful as the pellets facilitate Salmonella colonization by stimulating the secretion of mucins (Hedemann et al., 2005).
For sanitation of the feed, we offer organic acids (Acidomix product range) or mixtures of organic acids and formaldehyde in countries where formaldehyde products are allowed (Formycine) to decrease the pathogenic load of the feed materials. In vitro trials show the effectiveness of the products:
For the in vitro trial with Formycine, autoclaved feed samples were inoculated with Salmonella enteritidis serovar Typhimurium DSM 19587 strain to reach a Salmonella contamination of 106 CFU/g of feed. After incubating at room temperature for three hours, Formycine Liquido was added to the contaminated feed samples at 0, 500, 1000, and 2000 ppm. The control and inoculated feed samples were further incubated at room temperature, and Salmonella counts (CFU/g) were carried out at 24, 48, 72 hours and on day 15. The limit of Salmonella detection was set at 100 CFU/g (102). Results are shown in figure 2.
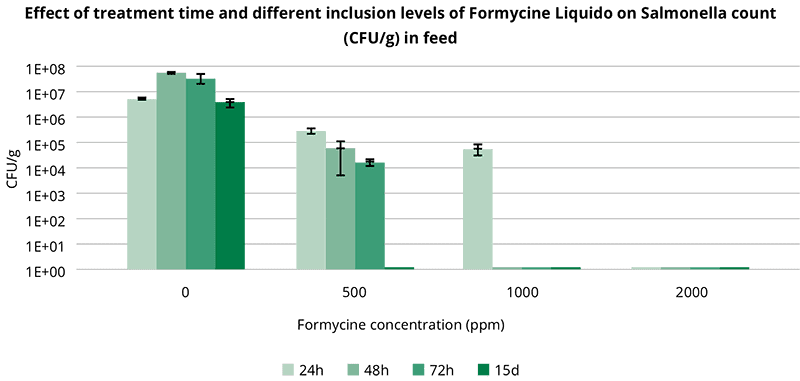 Fig. 2: Effect of treatment time and different inclusion levels of Formycine Liquido on the Salmonella count in feed
Fig. 2: Effect of treatment time and different inclusion levels of Formycine Liquido on the Salmonella count in feed
As important as uncontaminated feed is clean water for drinking. It can be achieved by taking the water from a main or a bacteriologically controlled water borehole. Regular cleaning/disinfection of the tanks, pipes, and drinkers is essential.
Bedding should be Salmonella-free
Straw material containing feces of other animals (rodents, pets) always carries the risk of Salmonella contamination. Also, wet or moldy bedding is not recommended because it is an additional challenge for the animal. To optimize the quality of bedding, the straw should be bought from reliable and as few as possible sources. The material must be stored dry and as far as practicable from the pig buildings (Ministry of Agriculture, Fisheries and Food & Scottish Executive Rural Affairs Department, 2000).
Vaccination is a beneficial measure
For the control of Salmonella in swine herds, vaccination is an effective tool. De Ridder et al. (2013) showed that an attenuated vaccine reduced the transmission of Salmonella Typhimurium in pigs. The vaccination with an attenuated S. Typhimurium strain, followed by a booster vaccination with inactivated S. Cholerasuis, showed better effects than an inactivated S. Cholerasuis vaccine alone (Alborali et al., 2017). Bearson et al. (2017) could delimitate transmission through less shedding and protect the animals against systemic disease.
To achieve the best effects, the producer must understand the diversity of Salmonella serovars to choose the most promising vaccination strategy (FSIS, 2023).
2. How to minimize the spreading of Salmonella on the farm?
If there are already cases of Salmonella on the farm, infected animals must be separated from the rest of the herd. Small batch sizes are beneficial, as well as not mixing different litters after weaning. If feasible, separate units for different production phases with an all-in/all-out system could break the reinfection cycle and help reduce Salmonella contamination on the farm. And also in this case, vaccination is helpful.
Salmonella doesn’t like acid conditions
An effective tool is acidifying the feed with organic acids, as Salmonella doesn’t like acid conditions. A trial was conducted with Acidomix AFG and Acidomix AFL to show their effects against Salmonella. For the test, 105 CFU/g of Salmonella enterica ser. Typhimurium was added to feed containing 1000 ppm, 2000 ppm, and 3000 ppm of Acidomix AFG or AFL. The stomach and intestine were simulated in vitro by adjusting the pH with HCl and NaHCO3 as follows:
Stomach 2.8
Intestine 6.8-7.0
After the respective incubation, the microorganisms were recovered from feed and plated on an appropriate medium for CFU counting. The results are shown in figures 3 and 4.
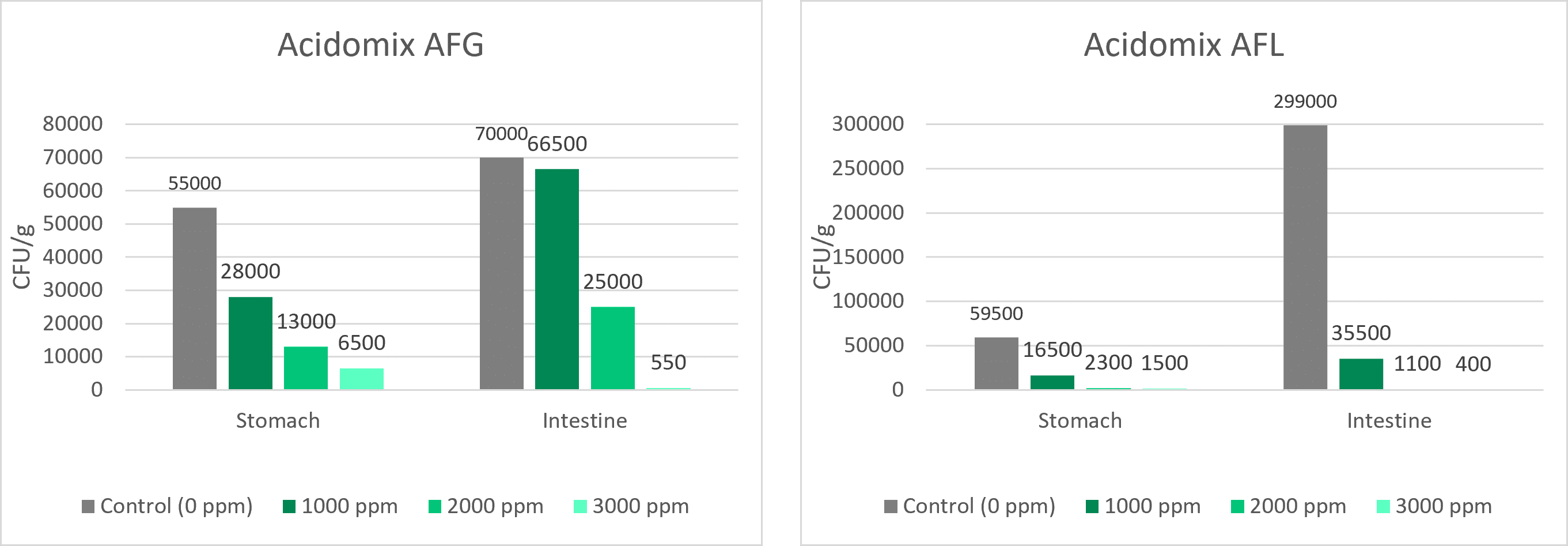
Figures 3 + 4: Effects of different concentrations of Acidomix AFG and Acidomix AFL against Salmonella enterica ser. Typhimurium in feed
Phytomolecules can support pigs against Salmonella
Plant compounds or phytomolecules can also be used against Salmonella in pigs. Some examples of phytomolecules to be used are Piperine, Allicin, Eugenol, and Carvacrol. Eugenol, e.g., increases the permeability of the Salmonella membrane, disrupts the cytoplasmic membrane, and inhibits the production of bacterial virulence factors (Keita et al., 2022; Mak et al., 2019). Thymol and Carvacrol interact with the cell membrane by H bonding, also resulting in a higher permeability.
An already published in vitro trial conducted with our product Ventar D also showed excellent effects against Salmonella while sparing the beneficial gut flora. A further trial once more demonstrated the susceptibility of Salmonella to Ventar D. It showed that Ventar D controls Salmonella by suppressing their motility and, at higher concentrations, inactivating the cells (see figures 5 + 6):
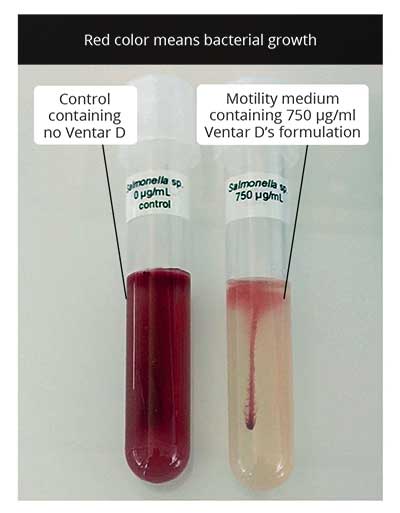 Figure 5: S. enterica motility test: on the left side – control; on the right side – motility medium containing.750 µg/mL of Ventar
Figure 5: S. enterica motility test: on the left side – control; on the right side – motility medium containing.750 µg/mL of Ventar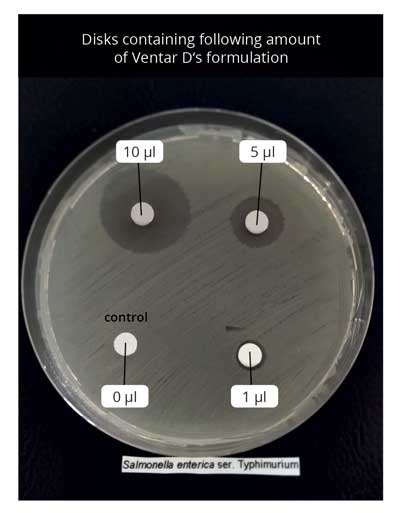 Fig 6 . Disk diffusion assay employing S. enterica. upper left side – disk containing 10 µL of Ventar; upper right – 5 µL; lower left – control; lower right – 1µL.
Fig 6 . Disk diffusion assay employing S. enterica. upper left side – disk containing 10 µL of Ventar; upper right – 5 µL; lower left – control; lower right – 1µL.In addition to the direct Salmonella-reducing effect, essential oils / secondary plant compounds / phytomolecules improve digestive enzyme activity and digestion, leading to increased nutrient absorption and better feed conversion (Windisch et al., 2008).
3. How can the farmer keep Salmonella contamination low in the slaughterhouse?
In general, the slaughterhouse personnel is responsible for adequate hygiene management to prevent contamination of carcasses and meat. However, also the farmer can make his contribution to maintain the risk of contamination in the slaughterhouse as low as possible. A study by Vieira-Pinto (2006) revealed that one Salmonella-positive pig can contaminate several other carcasses.
According to a trial conducted by Hurd et al. (2002), infection and, therefore, “contamination” of other pigs can rapidly occur, meaning that cross-contamination is a topic during transport to the slaughterhouse and in the lairages when the pigs come together with animals from other farms. The stress to which the pigs are exposed influences physiological and biochemical processes. The microbiome and animal’s immunity are affected, leading to higher excretion of Salmonella during transport and in the lairages. So, the animals should not be stressed during loading and unloading or transportation. The trailer poses a further risk of infection if it was not cleaned and disinfected before. So, reliable people who treat the animals well and keep their trailers clean should be chosen for transportation.
Pig producers are obliged to keep Salmonella in check – phytomolecules can help
At least in the EU, pig producers have the big duty to keep Salmonella low in their herds; otherwise, they will have financial losses. They are not only responsible for their farm, but also the slaughterhouses count on them. Besides the standard strict hygiene management and vaccination, farmers can use products provided by the industry to sanitize feed but also to support their animals directly with phytomolecules acting against pathogens and supporting gut health.
All these measures together should be a solution to the immense challenge of Salmonella, to protect people and prevent economic losses.
References:
Alborali, Giovanni Loris, Jessica Ruggeri, Michele Pesciaroli, Nicola Martinelli, Barbara Chirullo, Serena Ammendola, Andrea Battistoni, Maria Cristina Ossiprandi, Attilio Corradi, and Paolo Pasquali. “Prime-Boost Vaccination with Attenuated Salmonella Typhimurium Δznuabc and Inactivated Salmonella Choleraesuis Is Protective against Salmonella Choleraesuis Challenge Infection in Piglets.” BMC Veterinary Research 13, no. 1 (2017): 284. https://doi.org/10.1186/s12917-017-1202-5.
Balaji, R, K J Wright, C M Hill, S S Dritz, E L Knoppel, and J E Minton. “Acute Phase Responses of Pigs Challenged Orally with Salmonella Typhimurium.” Journal of Animal Science 78, no. 7 (2000): 1885. https://doi.org/10.2527/2000.7871885x.
Bearson, Bradley L, Shawn M. Bearson, Brian W Brunelle, Darrell O Bayles, In Soo Lee, and Jalusa D Kich. “Salmonella Diva Vaccine Reduces Disease, Colonization, and Shedding Due to Virulent S. Typhimurium Infection in Swine.” Journal of Medical Microbiology 66, no. 5 (2017): 651–61. https://doi.org/10.1099/jmm.0.000482.
Brenner Michael, G, M Cardoso, and S Schwarz. “Molecular Analysis of Salmonella Enterica Subsp. Enterica Serovar Agona Isolated from Slaughter Pigs.” Veterinary Microbiology 112, no. 1 (2006): 43–52. https://doi.org/10.1016/j.vetmic.2005.10.011.
Chen, P.-L., C.-M. Chang, C.-J. Wu, N.-Y. Ko, N.-Y. Lee, H.-C. Lee, H.-I. Shih, C.-C. Lee, R.-R. Wang, and W.-C. Ko. “Extraintestinal Focal Infections in Adults with Non-typhoid Salmonella Bacteraemia: Predisposing Factors and Clinical Outcome.” Journal of Internal Medicine 261, no. 1 (2007): 91–100. https://doi.org/10.1111/j.1365-2796.2006.01748.x.
Chiu, Cheng-Hsun, Lin-Hui Su, and Chishih Chu. “Salmonella EntericaSerotype Choleraesuis: Epidemiology, Pathogenesis, Clinical Disease, and Treatment.” Clinical Microbiology Reviews 17, no. 2 (2004): 311–22. https://doi.org/10.1128/cmr.17.2.311-322.2004.
De Ridder, L., D. Maes, J. Dewulf, F. Pasmans, F. Boyen, F. Haesebrouck, E. Méroc, P. Butaye, and Y. Van der Stede. “Evaluation of Three Intervention Strategies to Reduce the Transmission of Salmonella Typhimurium in Pigs.” The Veterinary Journal 197, no. 3 (2013): 613–18. https://doi.org/10.1016/j.tvjl.2013.03.026.
Deane, Annette, Declan Murphy, Finola C. Leonard, William Byrne, Tracey Clegg, Gillian Madigan, Margaret Griffin, John Egan, and Deirdre M. Prendergast. “Prevalence of Salmonella spp. in Slaughter Pigs and Carcasses in Irish Abattoirs and Their Antimicrobial Resistance.” Irish Veterinary Journal 75, no. 1 (2022). https://doi.org/10.1186/s13620-022-00211-y.
Edel, W., M. Schothorst, P. A. Guinée, and E. H. Kampelmacher. “Effect of Feeding Pellets on the Prevention and Sanitation of Salmonella Infections in Fattening Pigs1.” Zentralblatt für Veterinärmedizin Reihe B 17, no. 7 (2010): 730–38. https://doi.org/10.1111/j.1439-0450.1970.tb01571.x.
EFSA. “Salmonella.” European Food Safety Authority. Accessed August 7, 2023. https://www.efsa.europa.eu/en/topics/topic/salmonella.
Elbediwi, Mohammed, Daiwei Shi, Silpak Biswas, Xuebin Xu, and Min Yue. “Changing Patterns of Salmonella Enterica Serovar Rissen from Humans, Food Animals, and Animal-Derived Foods in China, 1995–2019.” Frontiers in Microbiology 12 (2021). https://doi.org/10.3389/fmicb.2021.702909.
Elnekave, Ehud, Samuel Hong, Alison E Mather, Dave Boxrud, Angela J Taylor, Victoria Lappi, Timothy J Johnson, et al. “Salmonella Enterica Serotype 4,[5],12:I:- In Swine in the United States Midwest: An Emerging Multidrug-Resistant Clade.” Clinical Infectious Diseases 66, no. 6 (2018): 877–85. https://doi.org/10.1093/cid/cix909.
FCC Consortium. “Final Report – Food Safety.” European Commission, 2010. https://food.ec.europa.eu/system/files/2016-10/biosafety_food-borne-disease_salmonella_fattening-pigs_slaughthouse-analysis-costs.pdf.
Ferrari, Rafaela G., Denes K. Rosario, Adelino Cunha-Neto, Sérgio B. Mano, Eduardo E. Figueiredo, and Carlos A. Conte-Junior. “Worldwide Epidemiology of Salmonella serovars in Animal-Based Foods: A Meta-Analysis.” Applied and Environmental Microbiology 85, no. 14 (2019). https://doi.org/10.1128/aem.00591-19.
“FSIS Guideline to Control Salmonella in Swine Slaughter and Pork Processing Establishments.” FSIS Guideline to Control Salmonella in Swine Slaughter and Pork Processing Establishments | Food Safety and Inspection Service. Accessed August 14, 2023. https://www.fsis.usda.gov/guidelines/2023-0003.
Gal-Mor, Ohad, Erin C. Boyle, and Guntram A. Grassl. “Same Species, Different Diseases: How and Why Typhoidal and Non-Typhoidal Salmonella Enterica Serovars Differ.” Frontiers in Microbiology 5 (2014). https://doi.org/10.3389/fmicb.2014.00391.
González-Santamarina, Belén, Silvia García-Soto, Helmut Hotzel, Diana Meemken, Reinhard Fries, and Herbert Tomaso. “Salmonella Derby: A Comparative Genomic Analysis of Strains from Germany.” Frontiers in Microbiology 12 (2021). https://doi.org/10.3389/fmicb.2021.591929.
Government of South Australia. Typhoid and paratyphoid – including symptoms, treatment, and prevention, April 3, 2022. https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/infectious+diseases/typhoid+and+paratyphoid/typhoid+and+paratyphoid+-+including+symptoms+treatment+and+prevention.
Hauser, Elisabeth, Erhard Tietze, Reiner Helmuth, Ernst Junker, Kathrin Blank, Rita Prager, Wolfgang Rabsch, Bernd Appel, Angelika Fruth, and Burkhard Malorny. “Pork Contaminated with Salmonella Enterica Serovar 4,[5],12:I:−, an Emerging Health Risk for Humans.” Applied and Environmental Microbiology 76, no. 14 (2010): 4601–10. https://doi.org/10.1128/aem.02991-09.
Health and Wellbeing; address=11 Hindmarsh Square, Adelaide scheme=AGLSTERMS.AglsAgent; corporateName=Department for. “Sa Health.” Typhoid and paratyphoid – including symptoms, treatment, and prevention, April 3, 2022. https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/infectious+diseases/typhoid+and+paratyphoid/typhoid+and+paratyphoid+-+including+symptoms+treatment+and+prevention.
Hedemann, M. S., L. L. Mikkelsen, P. J. Naughton, and B. B. Jensen. “Effect of Feed Particle Size and Feed Processing on Morphological Characteristics in the Small and Large Intestine of Pigs and on Adhesion of Salmonella Enterica Serovar Typhimurium DT12 in the Ileum in Vitro1.” Journal of Animal Science 83, no. 7 (2005): 1554–62. https://doi.org/10.2527/2005.8371554x.
Hendriksen, Susan W.M., Karin Orsel, Jaap A. Wagenaar, Angelika Miko, and Engeline van Duijkeren. “Animal-to-Human Transmission ofSalmonellaTyphimurium DT104A Variant.” Emerging Infectious Diseases 10, no. 12 (2004): 2225–27. https://doi.org/10.3201/eid1012.040286.
Keita, Kadiatou, Charles Darkoh, and Florence Okafor. “Secondary Plant Metabolites as Potent Drug Candidates against Antimicrobial-Resistant Pathogens.” SN Applied Sciences 4, no. 8 (2022). https://doi.org/10.1007/s42452-022-05084-y.
Ministry of Agriculture, Fisheries and Food, and Scottish Executive Rural Affairs Department. “Salmonella on Pig Farms – Code of Practice for the Prevention and Control Of.” ReadkonG.com, 2000. https://www.readkong.com/page/code-of-practice-for-the-prevention-and-control-of-5160969.
Morrow, W.E. Morgan, and Julie Funk. Ms. Salmonella as a Foodborne Pathogen in Pork. North Carolina State University Animal Science, n.d.
Soumet, C., A. Kerouanton, A. Bridier, N. Rose, M. Denis, I. Attig, N. Haddache, and C. Fablet. Report, Salmonella excretion level in pig farms and impact of quaternary ammonium compounds based disinfectants on Escherichia coli antibiotic resistance § (2022).
Thisyakorn, Usa. “Typhoid and Paratyphoid Fever in 192 Hospitalized Children in Thailand.” Archives of Pediatrics & Adolescent Medicine 141, no. 8 (1987): 862. https://doi.org/10.1001/archpedi.1987.04460080048025.
Ung, Aymeric, Amrish Y. Baidjoe, Dieter Van Cauteren, Nizar Fawal, Laetitia Fabre, Caroline Guerrisi, Kostas Danis, et al. “Disentangling a Complex Nationwide Salmonella Dublin Outbreak Associated with Raw-Milk Cheese Consumption, France, 2015 to 2016.” Eurosurveillance 24, no. 3 (2019). https://doi.org/10.2807/1560-7917.es.2019.24.3.1700703.
Vieira-Pinto, M, R Tenreiro, and C Martins. “Unveiling Contamination Sources and Dissemination Routes of Salmonella Sp. in Pigs at a Portuguese Slaughterhouse through Macrorestriction Profiling by Pulsed-Field Gel Electrophoresis.” International Journal of Food Microbiology 110, no. 1 (2006): 77–84. https://doi.org/10.1016/j.ijfoodmicro.2006.01.046.
Waldron, P. “Keeping Cows and Humans Safe from Salmonella Dublin.” Cornell University College of Veterinary Medicine, December 25, 2018. https://www.vet.cornell.edu/news/20181218/keeping-cows-and-humans-safe-salmonella-dublin.
Wang, J.-H., Y.-C. Liu, M.-Y. Yen, J.-H. Wang, Y.-S. Chen, S.-R. Wann, and D.-L. Cheng. “Mycotic Aneurysm Due to Non-Typhi Salmonella: Report of 16 Cases.” Clinical Infectious Diseases 23, no. 4 (1996): 743–47. https://doi.org/10.1093/clinids/23.4.743.
WHO. “Salmonella (Non-Typhoidal).” World Health Organization, February 20, 2018. https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal).
Windisch, W., K. Schedle, C. Plitzner, and A. Kroismayr. “Use of Phytogenic Products as Feed Additives for Swine and Poultry1.” Journal of Animal Science 86, no. suppl_14 (2008). https://doi.org/10.2527/jas.2007-0459.
Windisch, W., K. Schedle, C. Plitzner, and A. Kroismayr. “Use of Phytogenic Products as Feed Additives for Swine and Poultry1.” Journal of Animal Science 86, no. suppl_14 (2008). https://doi.org/10.2527/jas.2007-0459.