By Kouji Umeda, Production Director, EW Nutrition Japan
Calves are susceptible to infection by pathogens due to their immature congenital immunity. Bovine rotavirus and bovine coronavirus, pathogenic E. coli, Clostridium, Cryptosporidium, and Eimeria spp are the major pathogens of infectious diarrhea in calves less than one month of age. Bovine rotavirus, the most frequently detected in dairy and beef cattle, is responsible for approximately 40% of diarrhea cases. In addition, 60-70% of cases of diarrhea involving bovine rotavirus occur within the first two weeks of life. Symptoms include fever, anorexia, loss of energy, and acute yellow-white watery diarrhea after 12 to 36 hours post infection, which leads to dehydration and metabolic acidosis. In more severe cases, the disease can lead to death and is considered one of the most severe diarrhea-causing pathogens in newborn calves worldwide.
Rotavirus A is a major causative pathogen of diarrhea in calf
Rotaviruses belong to the family of Reoviridae and are classified into species A to J. The rotaviruses in bovines mainly belong to species A, B, and C, which are the leading infectious agents in cattle. Calf diarrhea is primarily caused by rotavirus A (RVA). This virus is transmitted orally through feces, bedding, utensils, or people contaminated with feces. Significant diarrhea caused by the virus is attributed to
- malabsorption due to the destruction of small intestinal epithelial cells and
- inhibition of water reabsorption by enterotoxin (NSP4) produced by rotaviruses.
Adult cattle and other host animals have an immune system that protects them from infection and the development of various pathogens. As RVA exists in different genotypes, the antibodies must be specifically against this genotype; otherwise, the virus-neutralizing activity, as well as protection against infection and pathogenesis, is significantly reduced.
The classic method to prevent RVA infection
Besides adequate sanitation in the production facilities, farmers try to “improve” the composition of the maternal colostrum by vaccinating the cow. For this purpose, the cows are inoculated with inactivated, previously isolated bovine RVA. However, the immunization of calves through colostrum may not be effective enough. It also may be difficult to prevent the spread of bovine RVA by barn hygiene alone due to the recent increase in the number of cattle being raised and moved from one farm to another.
Calf diarrhea feces contain G and P genotypes of bovine RVA
In general, the three most common G genotypes of bovine RVA detected in calf diarrhea are G6, G8, and G10, and the three most common P genotypes are P[1], P[5], and P[11]. Based on the results of the genotyping survey in Japan from 1987 to 2000 (Fig. 1) and the one from 2017 to 2020 (figure 2) (Animal Health Research Division of the National Institute of Agrobiological Sciences (NIAH) together with IRIG), the bovine RVA genotypes identified as prevalent and endemic in Japan in recent years were G6P[5], G6P[11], and G10P[11]. However, the percentage of genotypes detected differed among cattle breeds (Fig. 3A, Fig. 3B, Fig. 3C).
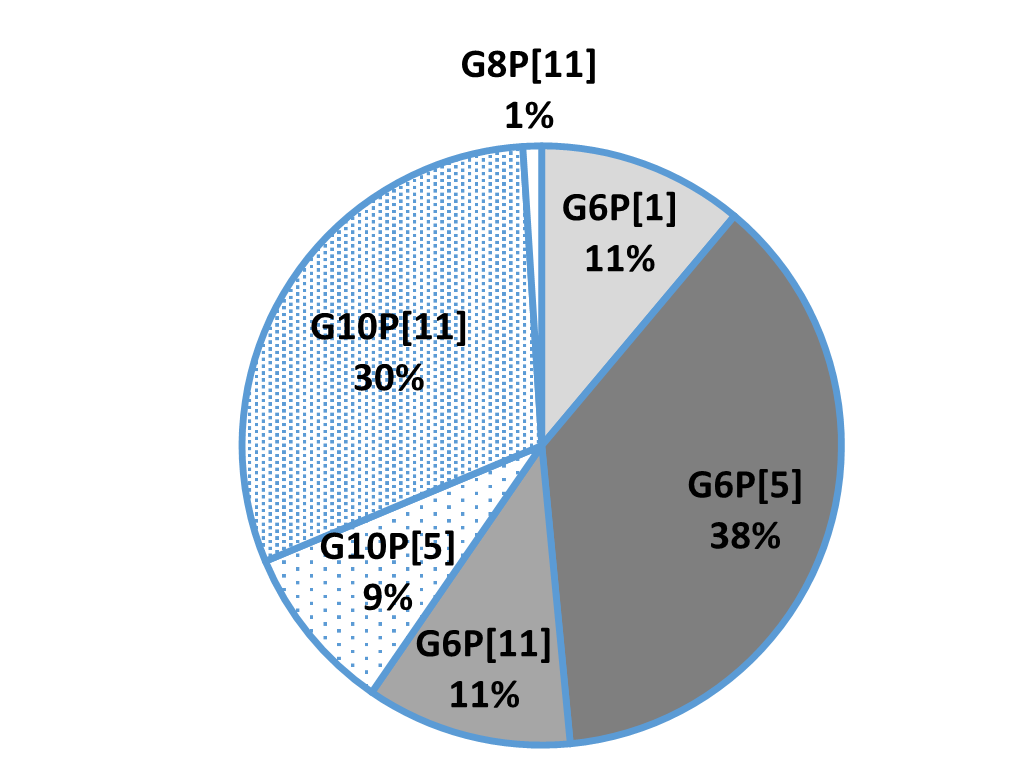 Fig.1: Genotyping results from 1987-2000
Fig.1: Genotyping results from 1987-2000
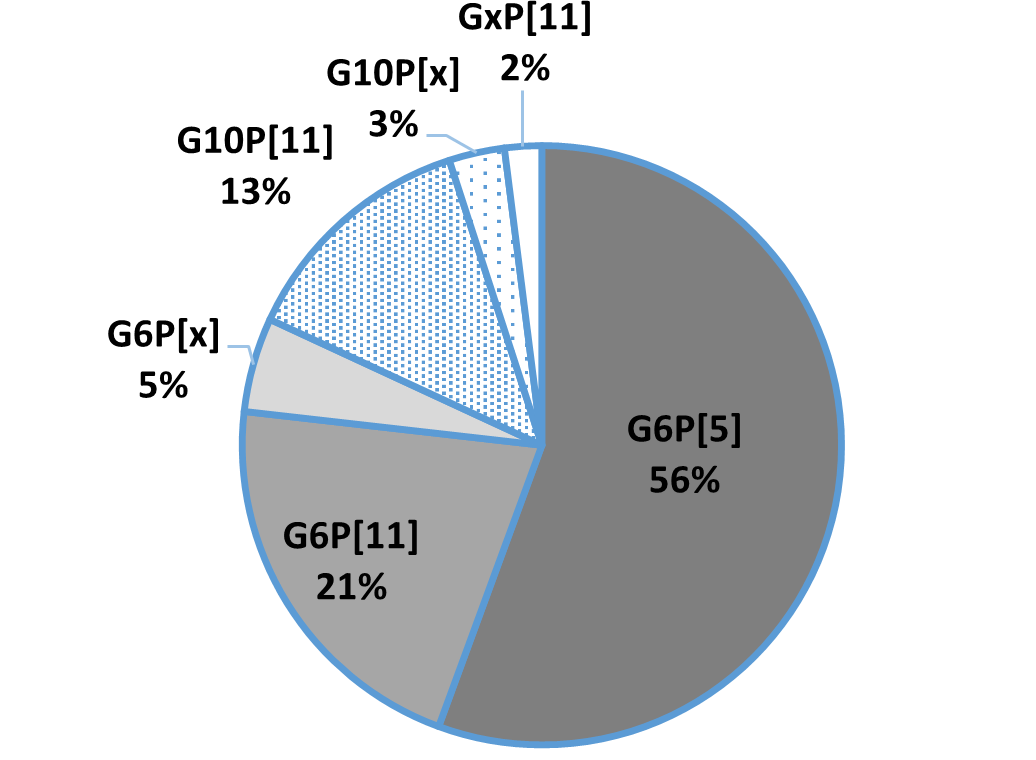 Fig.2: Genotyping results from 2017-2020
Fig.2: Genotyping results from 2017-2020
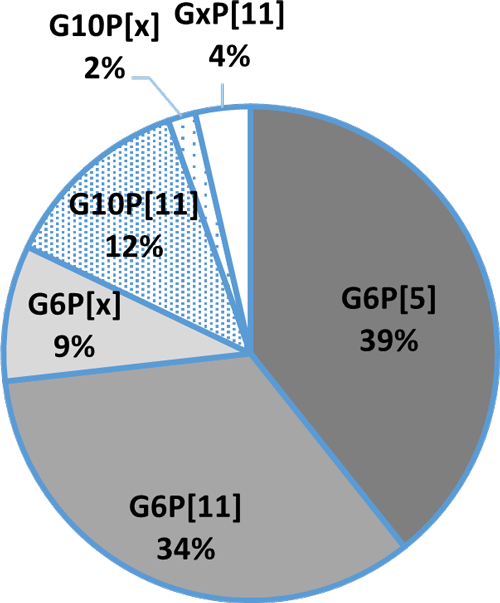 Fig. 3A:Percentage of detection in Holstein
Fig. 3A:Percentage of detection in Holstein
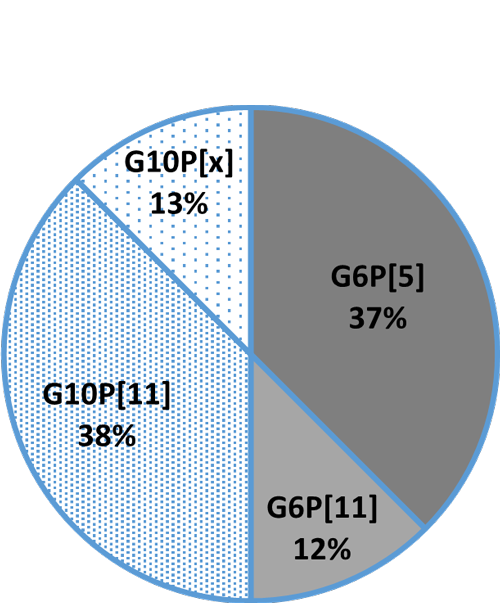 Fig. 3B: Detection rate in crossbreeds
Fig. 3B: Detection rate in crossbreeds Fig. 3C: Detection rate in beef cattle (Wagyu)
Fig. 3C: Detection rate in beef cattle (Wagyu)Cow colostrum protects the calf, egg yolk the chick AND the calf
A cow provides the calf with colostrum to ensure immunoglobulin delivery (passive immunity). In poultry, hens transfer immunoglobulins to the egg yolks and pass immunoglobulins to their chicks in this way. This biological mechanism of “immune transfer to the egg yolk” in birds can be used to arbitrarily produce yolk immunoglobulin (IgY) against pathogens of enteric infections in livestock (Ikemori et al., 1992; Ikemori et al., 1997; Yokoyama et al., 1998).

For this purpose, hens must get in contact with the respective pathogens. They produce antibodies against these pathogens – which also works with non-poultry-relevant pathogens such as bovine RVA – and transfer them to the egg (⇒IgY). The eggs with accumulated high levels of useful IgY can be collected almost daily. The immunoglobulins can be fed to livestock animals such as calves to protect them in critical times.
Continuous feeding of milk formulas containing IgY allows the IgY to remain in the intestinal lumen for a long time (Nozaki et al., 2019). There, they bind to the target pathogens and prevent infection by inhibiting their attachment to and cell invasion into intestinal epithelial cells.
IgY and genotype of the virus must match
A study verified that anti-bovine RVA IgY consisting of anti-G6P[1], anti-G6P[5], and anti-G10P[11] shows broad-spectrum virus-neutralizing activity against recent field isolates. Separate trials (see table 1) demonstrated that anti-G6 genotype IgY acted best against the RVA genotypes G6P[1] and G6P[5] and showed less activity against the G10 genotype. Anti-G10P[11] IgY worked optimally against the P[11] genotypes. The trials confirmed that either the G or the P genotype must match to achieve a sufficient virus-neutralizing activity. The IgY mixture is not helpful against bovine RVA strains that match neither the G nor the P genotypes (Odagiri et., 2020).
As the genotyping survey of 2017-2020 showed mainly G6 and G10 genotypes, a mixture of anti- bovine RVA G6P[1] IgY, G6P[5], and G10P[11] has strong virus neutralizing activity against bovine RVA that is currently prevalent and spreading in production sites.
Table 1: Virus-neutralizing activity of field-isolated bovine RVA against various genotypic strains
| IgY | Virus-neutralizing test strain | |||||||||
| SMN 1 | HKD 18 | SMN 35 | HKD 6 | HKD 7 | HKD 17 | KK-3 | OKY 31 | MYG 1 | Dai-10 | |
| 1978 | 2018 | 2018 | 2017 | 2017 | 2017 | 1983 | 2017 | 2017 | 2007 | |
| G6P[1] | G6P[5] | G6P[5] | G6P[11] | G6P[11] | G6P[11] | G10P[11] | G10P[11] | G8P[14] | G24P[33] | |
| anti-G6P[1] 1978 IgY | +++ | +++ | +++ | +++ | +++ | +++ | + | + | – | – |
| anti-G6P[5] 2018 IgY | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | – |
| Anti-G10P[11] 2017 IgY | + | + | + | + | ++ | ++ | +++ | +++ | – | – |
| Control IgY | – | – | – | – | – | – | – | – | – | – |
+++:Strong virus neutralizing activity; ++:Moderate virus neutralizing activity; +:Weak virus neutralizing activity; -:No virus neutralizing activity
Anti-bovine RVA IgY supports calves against rotavirus infection
To verify the protective effect of oral passive immunization with anti-bovine RVA IgY against bovine RVA infection, a trial with newborn calves was conducted.
Trial design: Eight calves were separated from their mothers immediately after birth without feeding colostrum and moved to a house with infected animals. From the first day, the calves were fed artificial milk supplemented with anti-bovine RVA IgY (n=4) or non-immune IgY (Control IgY; n=4) three times a day.
The parameters observed were fecal score, bovine RVA excretion, and weight gain; data were collected daily. The fecal score was calculated as the cumulative fecal score during the study period: 0 for normal stools, 1 for soft to muddy stools, and 2 for watery stools. Bovine RVA was isolated from daily fecal samples and evaluated by the total number of days of bovine RVA excretion.
Results: The anti-bovine RVA IgY group was found to be effective in reducing the incidence of diarrhea and shortening the duration of virus excretion in the infection test with the bovine RVA G6 genotype strain and the bovine RVA G10 genotype strain (tables 2 and 3).
Table 2: Efficacy of anti-bovine RVA IgY feeding in bovine RVA G6 genotype strain infection
| Test Group | Diarrhea incidence | Cumulative fecal score | Bovine RVA excretion days | Increase in body weight | |
| (n animals affected/n animals tested) | kg | % | |||
| Anti-bovine RVA IgY | 0% (0/4) | 0.0 ± 0.0* | 2.3 ± 0.5** | 1.3± 0.4** | 3.5 ± 0.7** |
| Control IgY | 100% (4/4) | 12.8 ± 4.8 | 7.8 ± 1.3 | – 3.3 ± 1.6 | – 7.6 ± 3.6 |
**: P<0.01; *: P<0.05
Table 3: Efficacy of anti-bovine RVA IgY feeding in bovine RVA G10 genotype strain infection
| Test Group | Diarrhea incidence | Cumulative fecal score | Bovine RVA excretion days | increase in body weight | |
| (n animals affected/n animals tested) | kg | % | |||
| Anti-bovine RVA IgY | 50% (2/4) | 2.3 ± 4.5** | 4.3 ± 1.3** | 1.1± 0.8** | 3.3 ± 3.1** |
| Control IgY | 100% (4/4) | 14.5 ± 3.7 | 7.3 ± 1.0 | – 4.2 ± 0.7 | – 11.1 ± 2.1 |
**: P<0.001
IgY is a valuable tool in rotavirus control
Newborn calves, susceptible to severe diarrhea caused by bovine RVA infection, require passive immunization with antibodies transferred from the colostrum of the mother cow. However, sometimes, calves don’t get enough antibodies which can be the case if
- the calf does not receive enough colostrum or receives it too late
- the cow still has not the farm-specific antibodies because of a too short time of being on the farm
To compensate for this lack of immunity, calves have been fed milk formulas containing anti-bovine RVA IgY for some time. Continuous feeding of anti-bovine RVA IgY, which shows strong virus neutralizing activity against each genotype of bovine RVA isolated from recent cases of calf diarrhea, is expected to provide sufficient immunity and be an effective means of bovine RVA control.
In the case of disease outbreaks, it makes sense to utilize IgY with appropriate mechanisms of action in addition to improving the level of quarantine measures, including hygiene control and vaccination.
References:
Ikemori, Yutaka, Masahiko Kuroki, Robert C. Peralta, Hideaki Yokoyama, and Yoshikatsu Kodama. “Protection of Neonatal Calves against Fatal Enteric Colibacillosis by Administration of Egg Yolk Powder from Hens Immunized with K99-Piliated Enterotoxigenic Escherichia Coli.” Amer. J. Vet. Res. 53, no. 11 (1992): 2005–8. PMID: 1466492.
Ikemori, Yutaka, Masashi Ohta, Kouji Umeda, Faustino C. Icatlo, Masahiko Kuroki, Hideaki Yokoyama, and Yoshikatsu Kodama. “Passive Protection of Neonatal Calves against Bovine Coronavirus-Induced Diarrhea by Administration of Egg Yolk or Colostrum Antibody Powder.” Veterinary Microbiology 58, no. 2-4 (1997): 105–11. https://doi.org/10.1016/s0378-1135(97)00144-2.
Nozaki, I., M. Itoh, F. Murakoshi, T. Aoki, K. Shibano, and K. Yamada. “Effect of an Egg Yolk Immunoglobulin(Igy)Product on Oocyst Shedding and Blood and Fecal Igy Concentrations in Cryptosporidium-Infected Calves.” Japanese Journal of Large Animal Clinics 10, no. 2 (2019): 68–72. https://doi.org/10.4190/jjlac.10.68.
Odagiri, Koki, Nobuki Yoshizawa, Hisae Sakihara, Koji Umeda, Shofiqur Rahman, Sa Van Nguyen, and Tohru Suzuki. “Development of Genotype-Specific Anti-Bovine Rotavirus a Immunoglobulin Yolk Based on a Current Molecular Epidemiological Analysis of Bovine Rotaviruses a Collected in Japan during 2017–2020.” Viruses 12, no. 12 (2020): 1386. https://doi.org/10.3390/v12121386.
Yokoyama, Hideaki, Robert C. Peralta, Kouji Umeda, Tomomi Hashi, Faustino C. Icatlo, Masahiko Kuroki, Yutaka Ikemori, and Yoshikatsu Kodama. “Prevention of Fatal Salmonelosis in Neonatal Calves, Using Orally Administered Chicken Egg Yolk Salmonella-Specific Antibodies.” Amer. J. Vet. Res. 59, no. 4 (1998): 416–20. PMID: 9563623.