Contamination with multiple mycotoxins is the rule for animal feeds, rather than the exception. Trial data shows that producers can prevent negative effects on animal health and performance by using high-performing toxin binders.

Multiple mycotoxins contaminate animal feed – problems and solutions
Mycotoxins pose an exceptional challenge for feed and animal producers. Generated by common molds, they occur in a great variety and numbers. Difficult to diagnose, mycotoxicosis in farm animals shows in a range of acute and chronic symptoms: decreased performance, feed refusal, poor feed conversion, reduced body weight gain, immune suppression, reproductive disorders, and residues in animal food products.
Regulatory mycotoxin thresholds don’t account for interactions
Regulatory thresholds for permissible mycotoxin levels in feed are derived from toxicological data on the effects of exposure of a certain species, at a certain production stage, to a single mycotoxin. This makes practical sense: while aflatoxins are carcinogens, fumonisins attack the pulmonary system in swine, for example. Mycotoxins also affect poultry in a different way than cattle, and broilers in a different way than breeders or laying hens, to mention more cases.
The problem is that, in reality, individual mycotoxin challenges are the exception. Animal diets are usually contaminated by multiple mycotoxins at the same time (Monbaliu et al., 2010; Pierron et al., 2016). Since 2014, EW Nutrition has conducted more than 50,000 mycotoxin tests on both raw material and finished feeds samples, across the globe. 85% of these samples were contaminated with more than one mycotoxin and one third positive for four or more mycotoxins.
How does contamination with multiple mycotoxins occur in animal feed?
The concurrent appearance of mycotoxins in feed can be explained as follows: each mold species has the capacity to produce several mycotoxins simultaneously. Each species, in turn, may infest several raw materials, leaving behind one or more toxic residue. In the end, a complete diet is made up of various raw materials with individual mycotoxin loads, resulting in a multitude of toxic challenges for the animals.
If animals were exposed to only one mycotoxin at a time, following the regulatory guidelines on maximum challenge levels would usually be enough to keep them safe. However, several studies have shown that the effects of exposure to multiple mycotoxins can differ greatly from the effects observed in animals exposed to a single mycotoxin (Alassane-Kpembi et al., 2015 & 2017). The simultaneous presence of mycotoxins may be more toxic than one would predict based on the known effects of the individual mycotoxins involved. This is because mycotoxins interact with each other. The interactions can be classified into three main different categories: antagonistic, additive, and synergistic (Grenier and Oswald, 2011).
Types of mycotoxin interactions
- Additivity occurs when the effect of the combination equals the expected sum of the individual effects of the two toxins.

- Synergistic interactions of two mycotoxins lead to a greater effect of the mycotoxin combination than would be expected from the sum of their individual effects. Synergistic actions may occur when the single mycotoxins of a mixture act at different stages of the same mechanism. A special form of synergy, sometimes called potentiation, occurs when one or both of the mycotoxins do not induce significant effects alone but their combination does. Fumonisin alone, for example, requires high levels to exerts effects on broiler performance. When aflatoxin is also in the feed, the effects are higher than those of aflatoxin alone (Miazzo et al., 2005)
- Antagonism can be observed when the effect of the mycotoxin combination is lower than expected from the sum of their individual effects. Antagonism may occur when mycotoxins compete with one another for the same target or receptor site. In an in-vitro study using human colon carcinoma cells (HCT116), Bensassi and collaborators (2014), found that DON and Zearalenone individually caused a marked decrease of cell viability in a dose-dependent manner; when combined, the effect was drastically reduced.
Most of the mycotoxin mixtures lead to additive or synergistic effects. The actual consequences for the animal will depend on its species, age, sex, nutritional status, the dose and duration of exposure as well as environmental factors. What is clear is that mycotoxin interactions pose a significant threat to animal health and critically impede risk assessment.
From awareness to action: risk assessment and toxin binders
Given their complex interactions, the toxicity of combinations of mycotoxins cannot merely be predicted based upon their individual toxicities. Mycotoxin risk assessments have to consider that even low levels of mycotoxin combinations can harm animal productivity, health, and welfare. Feed and animal producers need to be aware of which raw materials are likely to be contaminated with which mycotoxins, be able to accurately link them to the risk they pose for the animal and consequently take actions before the problems appear in the field.
Trials demonstrate effectiveness of toxin mitigation solutions
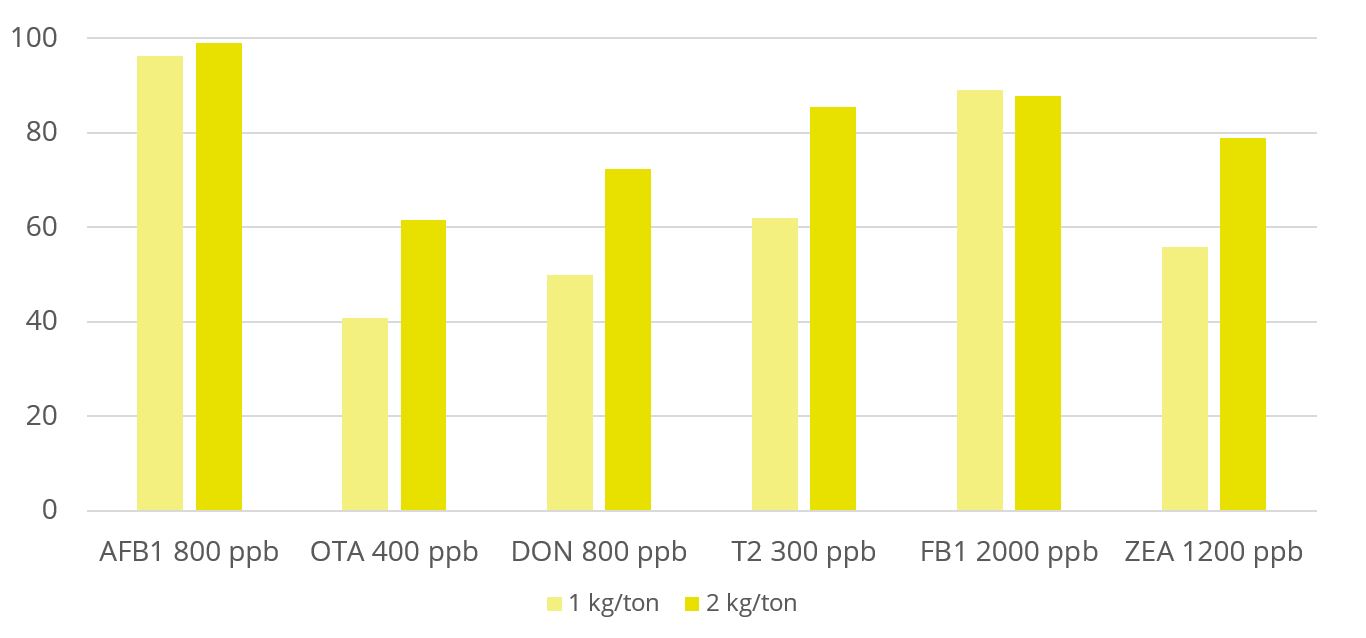
Toxin binders that are effective against a broad spectrum of mycotoxins significantly reduce the risks of mycotoxin exposure. In vitro trial data shows that EW Nutrition’s cost-effective toxin-mitigating product Solis Max shows a high mitigation capacity, even at low inclusion rates (Figure 1). Importantly, Solis Max helps to reduce various mycotoxins’ negative effects on performance without any negative effects on nutrient absorption.
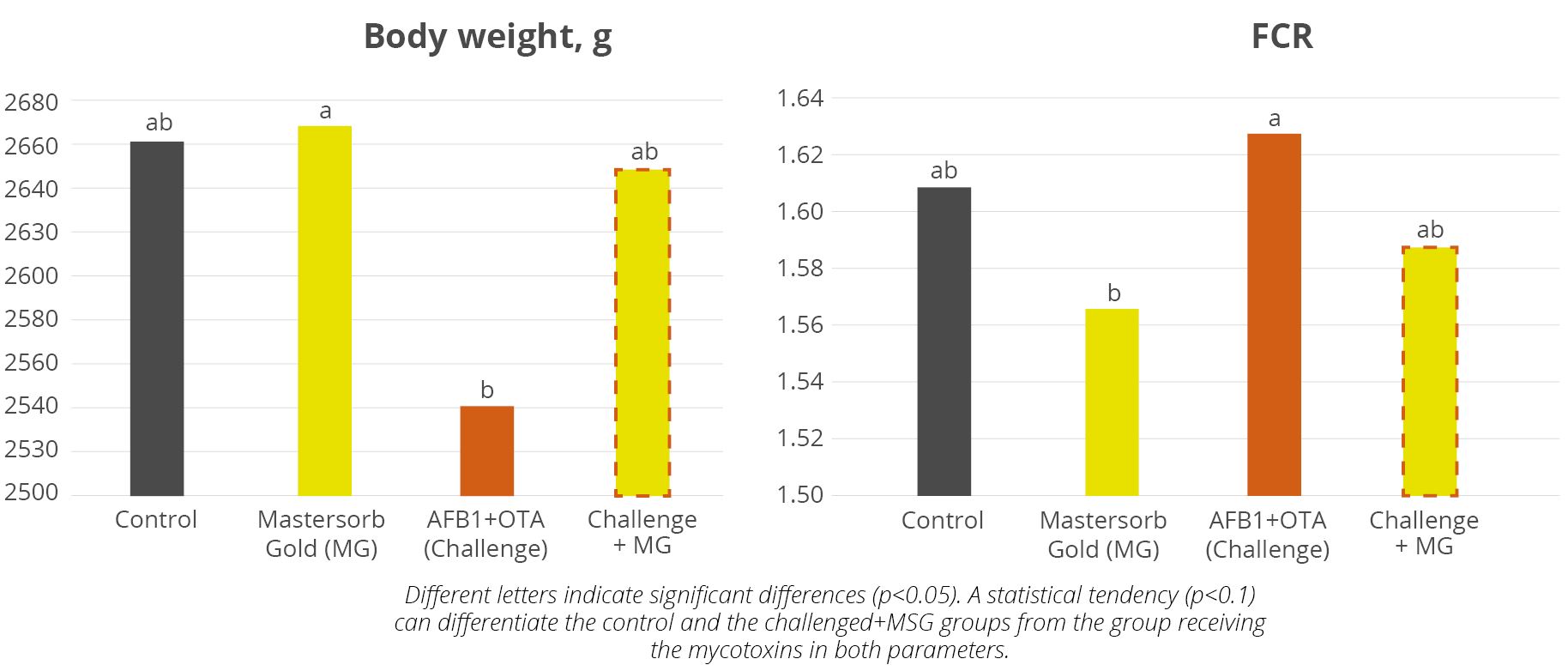
In a recent trial of 416 day-old Vencobb-430 broilers, premium product Mastersorb Gold has demonstrated its ability to support animals coping with multiple mycotoxin challenges. For broilers challenged with 200 ppb AFB1 and 350 ppb OTA, Mastersorb Gold supplementation resulted in 4.3% higher average daily weight gain than the challenged group, a higher body weight on day 42 and a 2% better feed conversion (Figure 2), which means a total recovery of the performance when compared with the non-challenged control.
Liver health also improved: after 21 days, broilers receiving Mastersorb Gold showed lower AST (-20%) and ALT (-50%) levels compared to the challenged group. Mycotoxin-induced stress was also lower, as evidenced by a 25% lower H/L ratio and 20% reduced white blood cell count for the Mastersorb Gold group. All of the mentioned biomarkers were similar to the non-challenged control, showing the preventive effects of Mastersorb Gold on health and performance.
Proactive management: tackle multiple mycotoxin challenges head on
Mycotoxins interactions are the norm, not the exception. Yet, regulatory standards currently only cover the effects of individual mycotoxins, leaving productions exposed to risks of additive and synergistic mycotoxin interactions animals’ health and performance. Luckily, management options are available: Careful risk evaluation explicitly includes the threat of multiple contaminations. And producers can proactively ensure better health, welfare and productivity of their animals by investing in the right toxin mitigation solution for their business.
References
Alassane-Kpembi, Imourana, Olivier Puel, and Isabelle P. Oswald. “Toxicological Interactions between the Mycotoxins Deoxynivalenol, Nivalenol and Their Acetylated Derivatives in Intestinal Epithelial Cells.” Archives of Toxicology 89, no. 8 (August 2015): 1337–46. https://doi.org/10.1007/s00204-014-1309-4.
Alassane-Kpembi, Imourana, Gerd Schatzmayr, Ionelia Taranu, Daniela Marin, Olivier Puel, and Isabelle Paule Oswald. “Mycotoxins Co-Contamination: Methodological Aspects and Biological Relevance of Combined Toxicity Studies.” Critical Reviews in Food Science and Nutrition 57, no. 16 (November 2017): 3489–3507. https://doi.org/10.1080/10408398.2016.1140632.
Bensassi, Fatma; Gallerne, Cindy; Sharaf el dein, Ossama; Rabeh Hajlaoui, Mohammed; Lemaire, Christophe and Bacha, Hassen. “In vitro investigation of toxicological interactions between the fusariotoxins deoxynivalenol and zearalenone” Toxicon 84 (2014): 1-6. https://doi.org/10.1016/j.toxicon.2014.03.005.
Grenier, B., and I. Oswald. “Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions.” World Mycotoxin Journal 4, no. 3 (May 5, 2011): 285–313. https://doi.org/10.3920/wmj2011.1281.
Miazzo, R., M.F. Peralta, C. Magnoli, M. Salvano, S. Ferrero, S.M. Chiacchiera, E.C.Q. Carvalho, C.A.R. Rosa, and A. Dalcero. “Efficacy of Sodium Bentonite as a Detoxifier of Broiler Feed Contaminated with Aflatoxin and Fumonisin.” Poultry Science 84, no. 1 (January 2005): 1–8. https://doi.org/10.1093/ps/84.1.1.
Monbaliu, Sofie, Christof Van Poucke, Christ’l Detavernier, Frédéric Dumoulin, Mario Van De Velde, Elke Schoeters, Stefaan Van Dyck, Olga Averkieva, Carlos Van Peteghem, and Sarah De Saeger. “Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method.” Journal of Agricultural and Food Chemistry 58, no. 1 (2010): 66–71. https://doi.org/10.1021/jf903859z.
Pierron, Alix, Imourana Alassane-Kpembi, and Isabelle P. Oswald. “Impact of Mycotoxin on Immune Response and Consequences for Pig Health.” Animal Nutrition 2, no. 2 (2016): 63–68. https://doi.org/10.1016/j.aninu.2016.03.001.