by Inge Heinzl, Editor, EW Nutrition
Lutein is a lipid-soluble pigment that can be found naturally in algae and plants. There, it is a component of the light-collecting complexes in the chloroplasts.
For example, kale contains a relatively high concentration of up to 0.25mg lutein per g wet weight. For industrial purposes, however, lutein is extracted from the petals of marigold; they contain up to 8.5mg/g wet weight.
In the animal organism, lutein occurs in the egg yolk, in milk, or the macula lutea (“yellow spot”) of the animal/human eye. However, animals and humans cannot synthesize it.
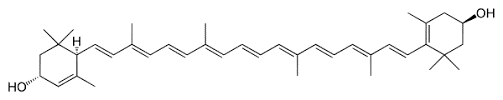
Lutein belongs to the group of carotenoids, which is divided into carotenes and xanthophylls. Lutein, chemically expressed as “3,3’-dihydroxy-α-carotene”, is a xanthophyll always accompanied by its isomer zeaxanthin. It is synthesized out of two α-carotenes through hydroxylation.
Lutein provides benefits for animals and humans
Due to its beneficial characteristics, lutein is an essential ingredient of plants and is used in animal nutrition as well as in human medicine.
Lutein has antioxidant protective properties
Under normal conditions, the cells in the animal and human organism control ROS (reactive oxygen species) levels. Usually, there is a balance between the generation of ROS and their elimination by scavenging systems. However, the high performance levels in modern animal production can easily lead to high ROS levels, translated into oxidative stress and leading to cell damage. Cell damage contributes to the generation of cancer and early aging in humans. In animals, the negative impact of oxidative stress can be responsible for lower performance and inferiority of meat and eggs.
Antioxidants stop ROS by taking up their energy
Through the uptake of energy, molecules can get into an excited state. One example is singlet excited oxygen, a highly reactive form of oxygen able to destroy proteins, lipids, and DNA. Carotenoids can intervene in this process: by exchanging electrons, the singlet excited oxygen gets neutralized, and the carotenoid gets into this excited state with higher energy. Once able to release this energy as heat into the environment, the carotenoid gets back to its normal state and can once again start acting as an antioxidant.
In this way, carotenoids, including lutein, ‘quench’ the energy of excited molecules and prevent the adverse effects of ROS (reactive oxidative substances).
Antioxidant properties profitably used
The antioxidant character of lutein plays an important role in the treatment or prophylaxis of macular degeneration in humans (Landrum & Bone, 2001). There is also evidence that lutein can be used to improve the visual and retinal function in dogs (Wang et al., 2016). In the eye, lutein and zeaxanthin, occurring in the retina and the macula, neutralize free radicals produced due to the ultraviolet light and thereby prevent damage to the macula.
Further possible applications are against cardiovascular diseases (Dwyer et al., 2001) and various types of cancer (e.g., breast cancer, Gong et al., 2018).
Lutein is important in infant nutrition
Lutein and its isomer zeaxanthin are the two primary carotenoids found in human milk (Giordano and Quadro, 2018). Stringham and co-workers (2019) postulate that lutein plays an important role in children’s visual and cognitive development/optimization. They report that a lutein supplementation of the mother can lead to a higher concentration of this substance in the milk and, consequently, in the child’s plasma (Sherry et al., 2014). In dairy cows, an increased level of lutein in the milk can also be observed (Xu et al., 2014), suggesting that lutein could also be essential in calf development.
Lutein stimulates the immune system
Another benefit of lutein is its positive influence on the immune system.
On the one hand, lutein stimulates the production of antibodies. In dogs, Guimarães Alarça et al. (2016) could show an increase of CD4+ and CD8+ T-lymphocyte subtypes. Kim et al. (2000) demonstrated the increase of lymphocytes and cells expressing CD5, CD4, CD8, and major histocompatibility complex class II (MHC II) molecules. Bédécarrats and Leeson (2006) provoked a higher secondary antibody response to infectious bronchitis vaccination in laying hens.
Besides, lutein acts as an anti-inflammatory agent, as shown in vitro by Chao et al. (2015) and in broiler chickens by Moraes and team (2016).
Lutein improves the attractivity of poultry products
In the marketing of poultry products, appearance and color are of central importance for evaluating quality. Egg yolk coloration is to a large extent a matter of regional preferences, however it is clear that an egg with a yolk that does not have the typical color is classified as inferior by the consumer. In areas with traditional corn growing, a white-skinned chicken is not commercially viable. Even when pullets are bought, the shanks and beaks should be yellow.
The use of xanthophylls like lutein and zeaxanthin enables producers to safely control the color of the egg yolk and of the broiler skin. It also leads to a healthy color of the shanks and beaks of the birds.
Lutein in a nutshell
Lutein is a true all-rounder: a substance that delivers benefits across the board. In plants, it helps fruits and petals become attractive for insects and other animals. It positively influences the animal, acting as an antioxidant, promoting infant development, and stimulating the immune system. As a pigment, it makes poultry and poultry products look more attractive to the consumer. Through its presence in eggs and milk, lutein provides clear and clean benefits to both animals and humans.
References
Bédécarrats, G.Y. and S. Leeson. “Dietary lutein influences immune response in laying hens.” J. Appl. Poult. Res. 15 (2006): 183–189.
https://doi.org/10.1093/japr/15.2.183
Chao, Shih-Chun, Tommaso Vagaggini, Chan-Wei Nien, Sheng-Chieh Huang, and Hung-Yu Lin. “Effects of Lutein and Zeaxanthin on LPS-Induced Secretion of IL-8 by Uveal Melanocytes and Relevant Signal Pathways.” Journal of Ophtalmology, vol. 2015 Article ID 152854 (2015): 7 pages. https://doi.org/10.1155/2015/152854
Dwyer, James H., Mohamad Navab, Kathleen M. Dwyer, Kholood Hassan, Ping Sun, Anne Shircore, Susan Hama-Levy, Greg Hough, Xuping Wang, Thomas Drake, C. Noel Bairey Merz, and Alan M. Fogelman. “Oxygenated Carotenoid Lutein and Progression of Early Atherosclerosis.” Circulation (American Heart Association) 103, no. 24 (2001): 2922-2927.
https://doi.org/10.1161/01.CIR.103.24.2922
Gong, Xiaoming, Joshua R. Smith, Haley M. Swanson, and Lewis P. Rubin. “Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms.” Molecules 23 no. 4(2018): 905.
http://dx.doi.org/10.3390/molecules23040905
Guimarães Alarça, Laís, Fabiane Yukiko Murakami, Ananda Portella Félix, Everton Luis Krabbe, Simone Gisele de Oliveira, Sebastião Aparecido Borges da Silva. “Dietary lutein supplementation on diet digestibility and blood parameters of dogs.” Cienc. Rural 46 no.12 (2016)
http://dx.doi.org/10.1590/0103-8478cr20151493
Kim, Hong Wook, Boon Chew, Teri Ann S Wong, Jean Soon Park, Bor-Chun Weng, Katherine M Byrne, Michael G Hayek, and Gregory A. Reinhart. “Dietary lutein stimulates immune response in the canine.” Veterinary Immunology and Immunopathology 74 no. 3-4 (2000): 315-327.
https://doi.org/10.1016/S0165-2427(00)00180-X
Landrum, J. T. and R.A. Bone. “Lutein, zeaxanthin, and the macular pigment.” Archives of Biochemistry and Biophysics 385 no. 1 (2001): 28–40.
https://doi.org/10.1006/abbi.2000.2171.
Moraes, M. L., A. M. L. Ribeiro, E. Santin, and K. C. Klasing. “Immunology, health, and disease: effects of conjugated linoleic acid and lutein on the growth performance and immune response of broiler chickens.” Poultry Science 95 (2016): 237–246.
http://dx.doi.org/10.3382/ps/pev325
Ochoa Becerra, Mario, Luis Mojica Contrerasa, Ming Hsieh Loa, Juan Mateos Díaz, Gustavo Castillo Herrera. “Lutein as a functional food ingredient: Stability and bioavailability.” Journal of Functional Foods 66 (2020): 103771.
https://doi.org/10.1016/j.jff.2019.103771
Sherry, Christina L., Jeffery S. Oliver, Lisa M. Renzi, and Barbara J. Marriage. “Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids.” J. Nutr. 144 (2014): 1256–1263.
http://dx.doi.org/10.3945/jn.114.192914
Stringham, James M., Elizabeth J Johnson, and B Randy Hammond. “Lutein across the lifespan: From childhood cognitive performance to the aging eye and brain.” Curr Dev Nutr 3 (2019): nzz066.
http://dx.doi.org/10.1093/cdn/nzz066
Wang, Wei, Jerome Hernandez, Cecil Moore, Janet Jackson, and Kristina Narfström. “Antioxidant supplementation increases retinal responses and decreases refractive error changes in dogs.” J. Nutr. Sci. 5 e18 (2016): 7 pages
http://dx.doi.org/10.1017/jns.2016.5
Xu, C.Z., H. F. Wang, J. Y. Yang, J. H. Wang, Z. Y. Duan, C. Wang, J. X. Liu , and Y. Lao. “ Effects of feeding lutein on production performance, antioxidative status, and milk quality of high-yielding dairy cows.” J. Dairy Sci. 97; American Dairy Science Association (2014):7144–7150
http://dx.doi.org/10.3168/jds.2014-8276