Author: Ajay Bhoyar, Senior Global Technical Manager, EW Nutrition
The global use of feed enzymes has become a central feature of efficient monogastric animal production systems. Rising feed ingredient costs, tighter margins, and increasing regulatory pressure to reduce environmental impact have all accelerated enzyme innovation. At the same time, feed mills have shifted toward higher conditioning temperatures and time in pursuit of improved pellet durability, pathogen control, and throughput. However, this creates a hostile environment for most exogenous feed enzymes, which can lose significant activity under the harsh conditions of feed processing.
Historically, enzyme manufacturers have attempted to overcome heat degradation of by coating, encapsulating, or post-pelleting liquid application (PPLA) of enzymes. While these approaches provide partial solutions, they can also have limitations, including delayed enzyme activity, uneven distribution, reduced mixing uniformity, and reliance on specialized liquid enzyme applicators.
These limitations prompted a novel direction: enzymes designed or selected to be intrinsically heat-stable, capable of surviving pelleting without protective matrices.
This article highlights recent advancements in intrinsically heat-stable xylanase technology, explains its advantages over coated and post-pelleting enzyme solutions, and outlines its practical benefits for feed manufacturers, integrators, and nutritionists operating under modern high-temperature feed pelleting conditions.
Intrinsically Thermostable Enzymes
An enzyme is considered intrinsically heat-stable when its native protein structure resists unfolding and retains catalytic activity under high temperatures associated with feed processing—typically 80–95°C for 30–90 seconds. Unlike coated enzymes that rely on external protection, intrinsically thermostable enzymes depend on their internal protein architecture for heat tolerance. Enzymes from organisms living in compost, thermal springs, and geothermal soils naturally withstand temperatures of 80–100 °C or higher. Intrinsically thermostable enzymes are often sourced from thermophiles (organisms living in hot springs and deep-sea vents) or engineered for stability. They resist denaturation (loss of shape and function) at high-temperature processing.
Limitations of Current Thermostability Solutions
Coating / Encapsulation
A method of protecting enzymes from heat is to encapsulate or coat them with a protective coating. An ideal enzyme coating for animal feed needs to:
1. Protect the enzyme through steam conditioning (typically 85–90°C or higher) and through subsequent pelleting.
2. Release the enzyme from the coating quickly in the gastrointestinal tract of the target animal, to ensure optimum efficacy. (Gilbert and Cooney, 2007)
There is some evidence, however, suggesting that the coating of enzymes may reduce the efficacy of the product, compared to an uncoated version of the same product (Kwakkel et al., 2000).
Post-Pelleting Liquid Application (PPLA)
Post-pelleting liquid enzyme application requires sophisticated applicators to minimize the risk of uneven spraying or calibration errors, which is often not feasible in small or mid-size mills. Accurate application of the liquid enzyme, as with some other critical liquid micro-ingredients, requires specialized spraying equipment and, even then, consistency of accurate enzyme application can be an issue (Bedford and Cowieson, 2009). Research has shown that as much as 30% of the enzyme activity can be found in the pellet fines, and therefore, adding the enzyme before screening would result in a lower than expected dosage in the final feed and wastage of the enzyme product (Engelen, 1998). In some cases, adjusting the pelleting machines to the output of the PPLA’s spray nozzles to ensure a homogenous and even application of the enzyme on the pellets may reduce the overall pellet production rate, especially in big feed mills with very high throughput.
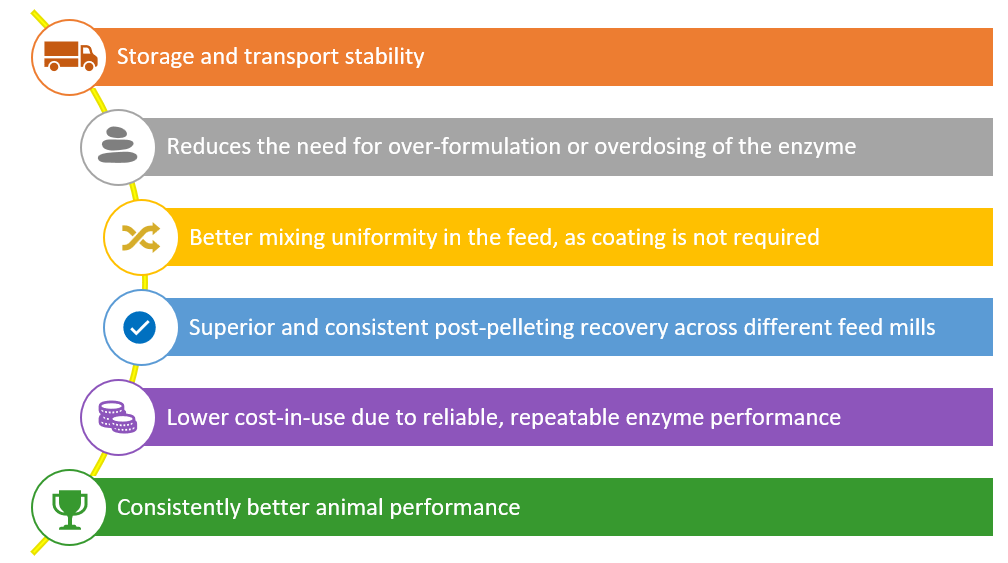
These limitations of the coated or PPLA technologies strengthen the value proposition of intrinsically heat-stable enzymes.
Nutritional and Commercial Benefits of Intrinsically Heat-Stable Xylanase
The use of intrinsically heat-stable xylanase delivers consistent nutritional benefits in poultry and swine feeds, including predictable non-starch polysaccharide (NSP) degradation, a significant increase in the metabolizable energy (ME) value of the feed, and enhanced gut health resilience supporting reduced antibiotic use.
From a commercial and operational perspective, this technology simplifies enzyme application, improves mixing uniformity, reduces formulation risk, and lowers feed cost per unit of meat or egg produced.
In-Vitro Thermal Stability Profile of Axxess XY
Axxess XY is a novel, intrinsically thermostable GH10 xylanase originating from Thermotoga maritima, a hyperthermophilic bacterium found in hydrothermal vents near volcanic grounds, and commercially it is produced by proprietary strain of Bacillus subtilis.
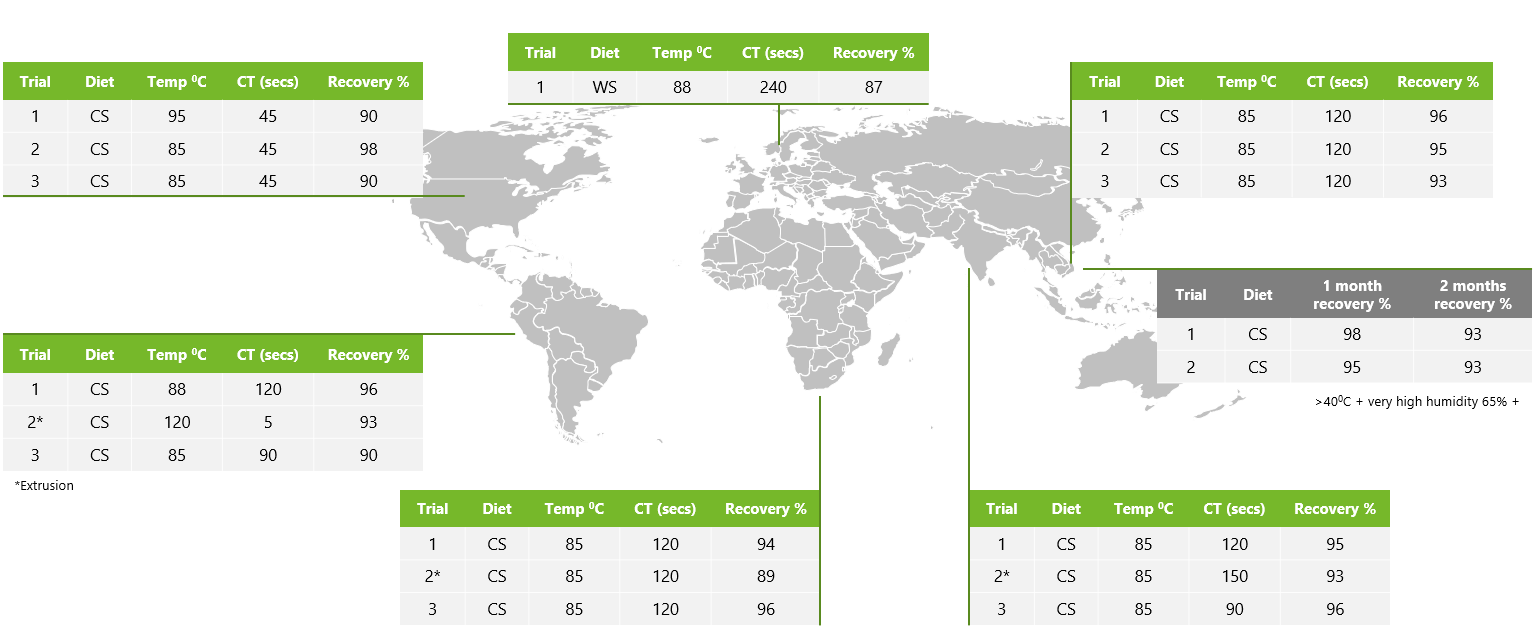
The superior heat stability of Axxess XY has been proven under various commercial pelleting conditions across different geographies. Axxess XY showed excellent post-pelleting recovery under commercial feed-milling conditions across varying temperatures and conditioning times (Fig. 2).
In one study, in addition to excellent post-pelleting recovery, Axxess XY also demonstrated high xylanase stability in pelleted feed over a 2-month feed storage period at>40°C, with humidity around 65%.
Conclusions
As feed mills continue to operate at higher conditioning temperatures and longer retention times, enzyme heat stability has become a critical success factor in modern feed production. Intrinsically heat-stable xylanase offers a practical and reliable solution to this challenge by maintaining enzyme activity through pelleting without the need for coatings or post-pelleting liquid application systems.
By relying on its native protein structure rather than external protection, intrinsically thermostable xylanase delivers consistent post-pelleting recovery, uniform distribution in feed, and predictable nutritional performance across different feed mills and processing conditions. This reliability translates into improved nutrient utilization, better gut health support, and reduced cost per kilogram of meat or eggs produced.
From an operational standpoint, intrinsically heat-stable xylanase simplifies enzyme application, reduces dependence on specialized equipment, and minimizes the need for over-formulation or safety margins. These advantages help feed manufacturers and integrators improve efficiency, lower risk, and achieve more consistent results, especially under demanding commercial pelleting conditions.
In summary, intrinsically heat-stable xylanase aligns well with the evolving needs of today’s feed industry, offering a robust, cost-effective, and future-ready enzyme solution for high-performance animal production systems.
References:
Bedford, M. R., and A. J. Cowieson. 2009. “Phytate and Phytase Interactions.” In Proceedings of the 17th European Symposium on Poultry Nutrition, 7–13. Edinburgh, UK.
Eeckhout, M., M. De Schrijver, and E. Vanderbeke. 1995. “The Influence of Process Parameters on the Stability of Feed Enzymes during Steam Pelleting.” In Proceedings of the 2nd European Symposium on Feed Enzymes, 163–169. Noordwijkerhout, The Netherlands.
Engelen, G. M. A. 1998. Technology of Liquid Additives in Post-Pelleting Applications. Wageningen, The Netherlands: Wageningen Institute of Animal Science.
Gilbert, T. C., and G. Cooney. 2011. “Thermostability of Feed Enzymes and Their Practical Application in the Feed Mill.” In Enzymes in Farm Animal Nutrition, 2nd ed., edited by M. R. Bedford and G. G. Partridge, 249–259. Wallingford, UK: CABI.
Kwakkel, R. P., P. L. van der Togt, and K. A. B. M. Holkenborg. 2000. “Bio-Efficacy of Two Phytase Formulations Supplemented to a Corn–Soybean Broiler Diet.” In Proceedings of the 3rd European Symposium on Feed Enzymes, 63–64. Noordwijkerhout, The Netherlands.