By Dr. Ajay Bhoyar, Global Technical Manager – Poultry, EW Nutrition
Rancidity testing is essential in the feed industry, as a key indicator of product quality and shelf life. It is conducted to determine the level of oxidation in samples of feed or feed ingredients and it can be performed through a number of analytical methods.
Rancidity is the process by which fats and oils in food become degraded, resulting into off-odor/flavor, taste, and texture. This process is caused by the oxidation of unsaturated fatty acids and can be accelerated by factors such as exposure to light, heat, and air. Rancidity can occur naturally over time, but it can also be accelerated by improper storage or processing of animal products. Fats are highly susceptible to degradation due to their chemical nature.
How does oxidative rancidity occur?
Oxidation occurs when an oxygen ion replaces a hydrogen ion within a fatty acid molecule and higher numbers of double bonds within the fatty acid increase the possibility of autoxidation. Oxidative rancidity results from the breakdown of unsaturated fatty acids in the presence of oxygen. Light and heat promote this reaction, which results in the generation of aldehydes and ketones – compounds which impart off-odors and flavors to food products. Pork and chicken fat demonstrate a higher degree of unsaturated fatty acids compared with beef fat and are therefore more prone for rancidity.
Oxidation: a three-step process
Fat/oil oxidation is a three-step process (Initiation, Propagation and Termination). Therefore, the oxidation products depend on the time. In the first phase, called Initiation, the formation of free radicals begins and accelerates.
Once the initial radicals have formed, the formation of other radicals proceeds rapidly in this second phase called Propagation. In this part of the process, a chain reaction of high energy molecules, which are variations of free radicals and oxygen, are formed and can react with other fatty acids. These reactions can proceed exponentially, if not controlled. Also in this phase, the rate of peroxide radical formation will reach equilibrium with the rate of decomposition to form a bell-shaped curve.
In the final phase, called Termination, the starting material has been consumed, and the peroxide radicals, as well as other radicals decompose into secondary oxidation by-products such as esters, short chain fatty acids, polymers, alcohols, ketones and aldehydes. It is these secondary oxidation by-products, which can negatively affect the growth and performance of animals.
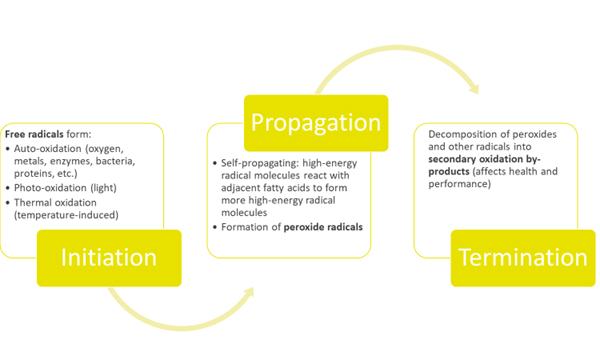
Fig. 1: Oxidation: a three-phase series of reactions
Antioxidants preserve the quality of rendered products
Chemical antioxidants are used in the rendering industry to help preserve the quality of animal by-products. Synthetic antioxidants, such as BHA, BHT, and ethoxyquin, can help prevent the oxidation of these by-products, which can cause them to become rancid. These chemical antioxidants are added in small amounts to the raw materials prior to rendering or can be incorporated into the finished products to help extend their shelf life and maintain their nutritional value. It is important to note that the use of antioxidants in the rendering industry must be done in compliance with regulations and guidelines set forth by the FDA and other governing bodies.
Natural antioxidants like tocopherols, rosemary extract, ascorbyl palmitate, etc. are also used to prevent oxidation and maintain the freshness of rendered products, if the chemical antioxidants cannot be used.
Rancidity testing
Rancidity testing is the process of determining the level of rancidity in a product. Testing for level of rancidity is used widely as an indication of product quality and stability.
There are several methods used for rancidity testing, including:
Organoleptic rancidity testing
Oxidation of fats and oils leads to a change in taste, smell, and appearance. Organoleptic testing involves using the senses (sight, smell, taste) to determine the level of rancidity. Trained testers will examine the product for visual signs of spoilage, such as discoloration or the presence of crystals, and will also smell and taste the product to detect any off-flavors or odors.
Chemical & instrumental rancidity testing
Chemical testing involves using chemical methods to measure the level of rancidity. One common method is the peroxide value test, which measures the amount of peroxides (indicators of rancidity) in the product. Another method is the p-anisidine test, which measures the level of aldehydes (another indicator of rancidity) in the product.
Peroxide value
Peroxide Value (PV) testing determines the amount of peroxides in the lipid portion of a sample through an iodine titration reaction targeting peroxide formations. Peroxides are the initial indicators of lipid oxidation and react further to produce secondary products such as aldehydes. Because peroxide formation increases rapidly during the early stages of rancidification but subsequently diminishes over time, it is best to pair PV testing with p-Anisidine Value to obtain a fuller picture of product quality.
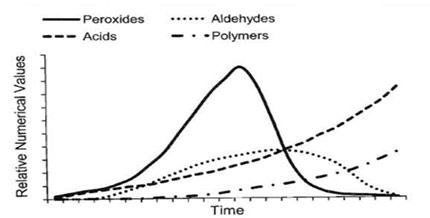
Fig.2: Oxidation products changes with time
p-Anisidine (p-AV)
p-AV is a determination of the amount of reactive aldehydes and ketones in the lipid portion of a sample. Both compounds can produce strong objectionable flavors and odors at relatively low levels. The compound used for this analysis (p-Anisidine) reacts readily with aldehydes and ketones and the reaction product can be measured using a colorimeter. Samples that are particularly dark may not be the most applicable for this analysis as the colorimeter may not be able to adequately measure the wavelength required.
TBARS
Thiobarbituric acid reactive substances (TBARS) are a byproduct of lipid peroxidation (i.e. as degradation products of fats). This can be detected by the TBARS assay using thiobarbituric acid as a reagent. TBA Rancidity (TBAR) also measures aldehydes (primarily malondialdehyde) created during the oxidation of lipids. This analysis is primarily useful for low-fat samples, as the whole sample can be analyzed rather than just the extracted lipids.
The Instrumental testing involves using instruments to measure the level of rancidity.
Gas chromatography
One common method is the use of a gas chromatograph, which can detect the presence of volatile compounds that indicate rancidity.
Fourier-transform infrared spectrophotometer (FTIR)
FTIR method can detect changes in the chemical makeup of the product that indicate rancidity.
Free Fatty Acids (FFA)
FFA testing determines the fatty acids that have been liberated from their triglyceride structure. A titration is performed on the extracted fat from a specific sample. The FFA content is then determined through a calculation of the amount of titrant used to reach the final result. Knowing what type of fat or fat containing product is being tested is important for this analysis to ensure that the appropriate calculation is applied. As the test does not differentiate between fatty acid types, samples with high palmitic or lauric fatty acid composition should have a different calculation factor applied so as to accurately represent the free fatty acid result.
Oxidative Stability Index (OSI)
OSI indicates how resistant a sample is to oxidation. Samples are subjected to heat while air is injected – a process which accelerates oxidation reactions. The samples are monitored, and the time required for the sample to reach an inflection point is determined. This test is useful when testing the efficacy of an antioxidant added to a product. Antioxidants should inhibit free radical propagation and thus increase a samples ability to hold up under the stressing conditions imposed by the OSI analysis. The measuring instrument, the Rancimat.
Analytical testing considerations in rendering operations
It is common to perform regular analytical testing in a rendering operation as a part of quality control and quality assurance program. There are several methods for testing rancidity in rendering operations. It is important to choose the appropriate method based on the type of product and the desired level of accuracy.
The results of rancidity testing are used to monitor and control the rendering process to prevent or minimize rancidity. This may involve adjusting processing conditions, using antioxidants, or implementing other measures to reduce oxidation.
| Test objective | Analysis | Remarks |
| Current state of oxidation |
|
|
| Potential for future oxidation | Oxidative Stability Index (OSI) | Analyze the stability of oil/fats |
| Residual antioxidant | Gas chromatography | Value decreases as the antioxidant gets sacrificed |
Table. 1: Analytical testing considerations for rendering
Conclusion
Rancidity is a common problem in rendered animal products. It can have detrimental effects on both the quality and safety of the product. It is caused by the oxidation of fats and oils, leading to the formation of harmful compounds such as free radicals and hydroperoxides. The best way to prevent rancidity is through proper storage, packaging, and handling techniques, as well as the use of antioxidants to slow down the oxidation process. It is important for manufacturers and consumers to be aware of the potential for rancidity in rendered animal products and take the necessary precautions to ensure the safety and quality of the product.
















