By Dr. Inge Heinzl, Editor, EW Nutrition
Salmonella infection in poultry is a problem for the producer because of the performance losses of his flock. At the same time, products of salmonella-contaminated animals pose a severe risk to human health. In the USA, Salmonellosis in poultry is estimated to cost $ 11.6 billion each year (Wernicki et al., 2017) and more than € 3 billion in the EU (Ehuwa, 2021). As the use of antibiotics needs to be reduced to keep them effective, Salmonella control in poultry requires new solutions. This article shows how organic acids and phytomolecules can help to fight this problematic disease.
Salmonellosis: what it is, how it works, and why it’s such a problem
Salmonellosis is a zoonosis, meaning that it can be easily transferred from animals to humans. The transfer can occur via different routes:
- Direct contact with an infected animal
- Handling or consuming contaminated animal products such as eggs or raw meat from pigs, turkeys, and chicken
- Contact with infected vectors (insects or pets) or contaminated equipment
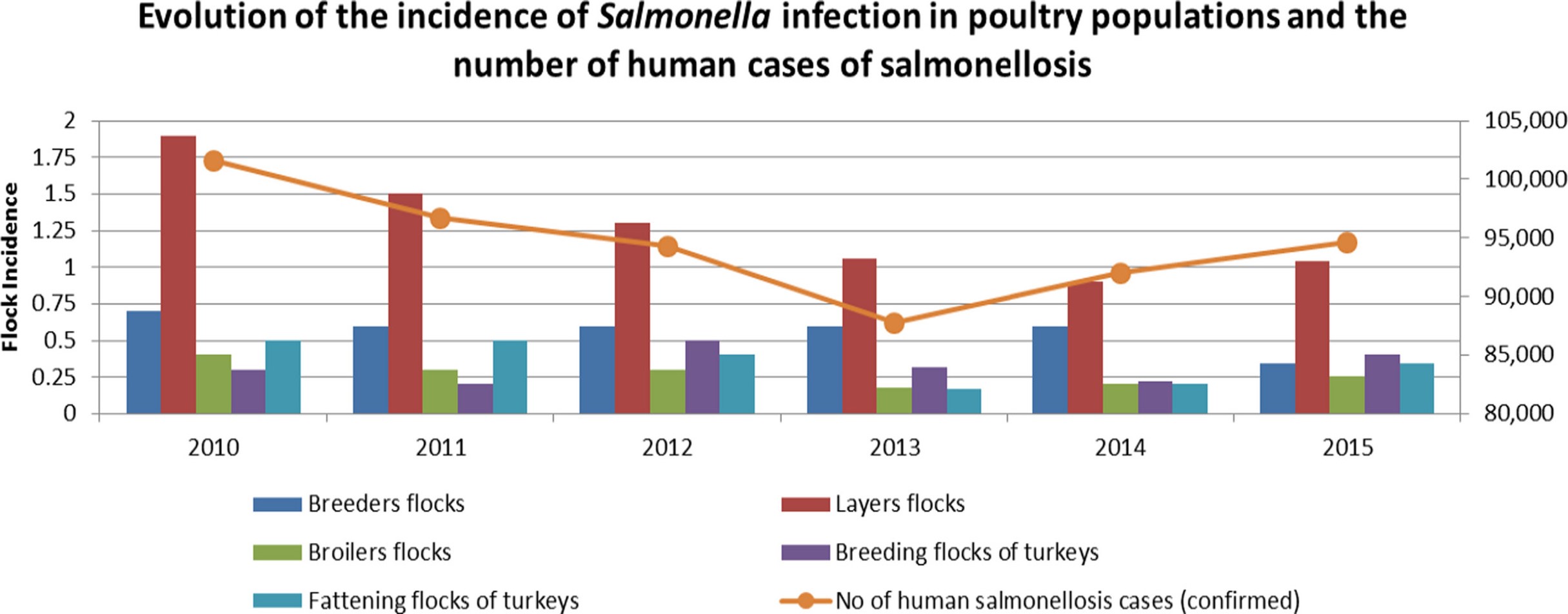
Frozen or raw chicken products, as well as the eggs of backyard hens, are the most frequent causes of animal-mediated Salmonella infections in humans. The following graphic shows a clear relationship between the occurrence of Salmonella in layer flocks and the event of disease in humans:
The impact of Salmonella on poultry depends on the bird’s age
Within the poultry flock, there are two ways of spreading: the fecal-oral way (horizontal infection) or the infection of the progeny in the egg (vertical infection). The effects of the disease depend on the age of the birds: the younger the animals, the more severe the impact.
If the brood eggs already carry salmonellae, the hatchability dwindles. During their first month of life, infected chicks show ruffled downs and higher temperatures. Diarrhea leads to fluid losses and frequently to the chicks’ death.
Adult animals usually do not die from Salmonellosis; often, the infection remains unnoticed. During a substantial acute salmonella outbreak, the animals show weakness and diarrhea. They lose weight, resulting in decreased egg production in layers and worse growth performance in broilers. The birds need more water to compensate for the fluid losses, and their crowns and jowls appear pale.
Salmonella protects itself through an intelligent infection style
Salmonellae have developed a clever way to protect themselves. After they arrive in the gut, they attach to the epithelial cells and form small molecular “syringes” to inject divers substances into the gut cells (Type-3-injection system). These signaling substances make the gut cells bulge their membranes and enclose the bacterium. Finally, the manipulated gut cell absorbs the Salmonella, the host “allows” the bacterium to enter, and it can proliferate in the gut cells (Fischer, 2018).
When an antibiotic is attacking the bacterium, Salmonellae stop their cell division. Since many antibiotics are only effective against bacteria during cell division and growth, Salmonellae survive the attack by staying as dormant variants or persisters until the treatment stops (Fischer, 2018).
Salmonellae – a big “family”
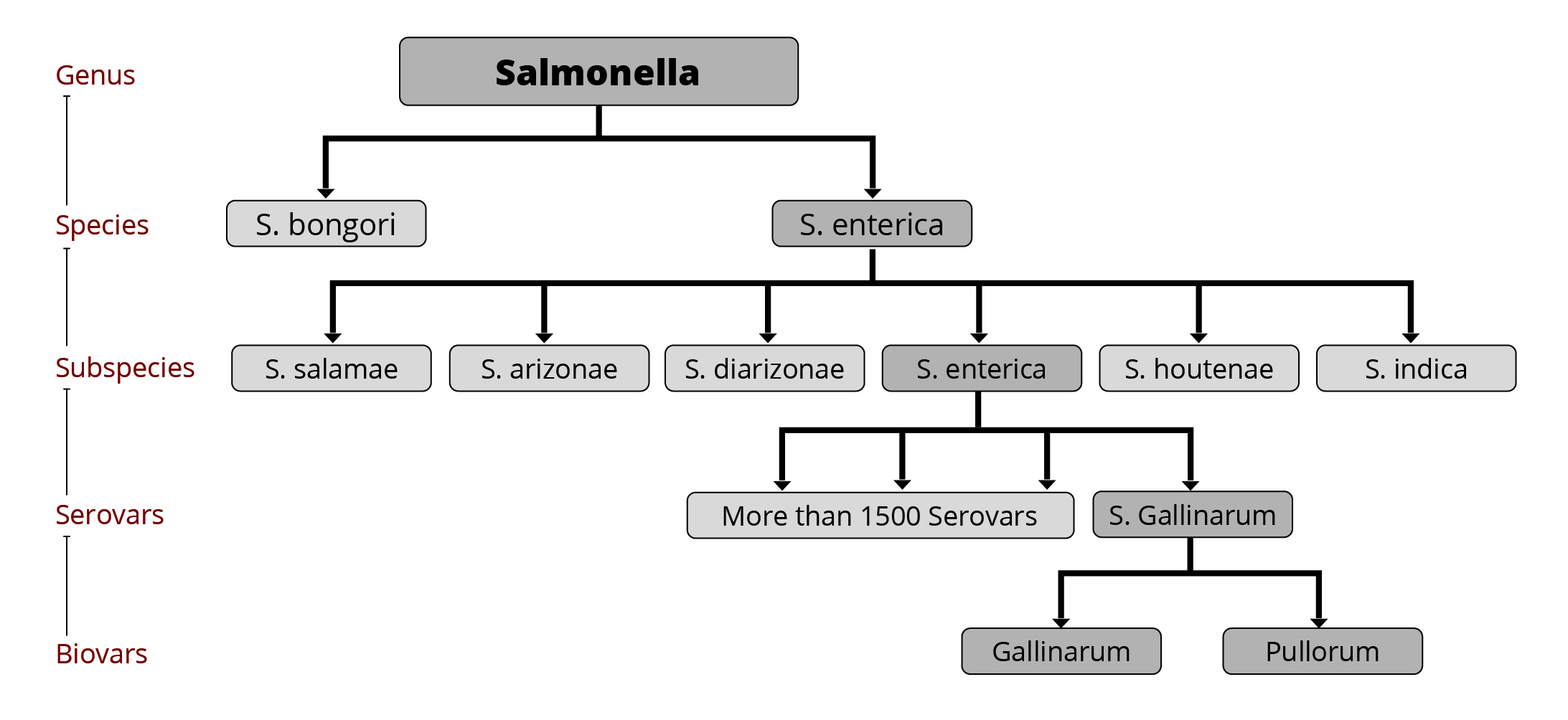
The genus of Salmonella consists of more than 2600 serovars (Ranieri et al., 2015), of which less than 100 are relevant for humans (CDC, 2020). More than 1500 serovars belong to the Salmonella enterica subspecies that colonize the intestinal tract of warm-blooded animals. These serovars are responsible for 99 % of salmonella infections (Mendes Maciel et al., 2017). The main serovars relevant for poultry are S. Gallinarum and S. Pullorum, but also S. Enteritidis, Typhimurium, and in recent years, S. Kentucky, S. Heidelberg, S. Livingstone, and S. Mbandaka (Guillén et al., 2020).
The zoonosis Salmonellosis must be controlled
Several Salmonella serovars are critical for animals and humans. Since more than 91,000 salmonellosis cases are reported for Europe and more than 1.35 million for the USA every year (EFSA, 2022; FDA, 2020), their spread must be prevented by all means. Governments have enacted some laws to curtail this disease. The EU, for example, implemented extended control programs for zoonotic diseases, with Salmonella set as a priority. These programs include the provision of scientific advice, targets for reducing Salmonella in poultry flocks, and restrictions on the trade of products from infected flocks.
For farmers and vets, this means the obligation to notify the occurrence of the disease to the authorities. Depending on the country, it also entails compulsory vaccination and the documentation of hygienic measures. In the EU, due to the risk of developing resistances, the EFSA recommends limiting the use of antimicrobials to individual cases, e.g., to prevent inordinate suffering of animals.
Prevention of Salmonella infection is the key
The best strategy for salmonella control is prevention based on three key points (Visscher, 2014):
- Preventing the introduction of Salmonella into the farm/flock through effective hygiene measures
- Preventing the spread of the pathogens within a flock/farm
- Prophylactic measures to recover immune resistance of the animals against Salmonella infection
For this purpose, the following steps are requested/recommended:
1. Keeping the litter dry
The use of well-absorptive material such as wood shavings, straw pellets, or straw granulates and regular removal of the used litter is recommended. The animals must be controlled for diarrhea to avoid wet droppings. The water supply must be adequate; an excessive water supply wets the litter.
2. Providing a clean environment
To keep the poultry house clean, broken eggs and dead animals (potential sources of infection) must be removed. In general, the houses should be cleaned and disinfected before every restocking.
Clean feed and water are essential; therefore, feed should not be stored outside but be kept dry and protected from pests and rodents. The feeding of the animals should take place inside to avoid contamination by wild birds. Concerning the water for drinking, the flow rate must be high enough to provide the birds with sufficient water but not too high that the floor gets wet. The troughs must be clean from droppings.
3. Limiting contacts
To limit the spread of Salmonella, only a restricted number of persons can have access to the flocks. They must wear clothes, and instruments should be exclusively used for the respective poultry house.
Knowing the optimal growth conditions for Salmonella facilitates control
Salmonellae are a genus in the family of Enterobacteriaceae. They are gram-negative, rod-shaped (size: approx. 2 µm), glucose-fermenting facultative anaerobes that are motile due to peritrichous flagella. Since Salmonellae do not form spores, they can be easily destroyed by heating them to 60°C for 15-20 min (Forsythe, 2001), especially in food/feed with higher water content.
For the storage of food, Bell and Kyriakis (2002) found that most serovars of Salmonella will not grow at temperatures lower than 7°C and a pH lower than 4.5. Wessels et al. (2021) showed optimal growth conditions for Salmonella: temperatures between 5 and 46°C (optimum 38°C), a water activity of 0.94-0.99, and a pH of 3.8-9.5.
A high fat content in the feed or food increases the likelihood of infection with Salmonella because the fat protects the bacteria during the passage through the stomach. Doses of 10 to 100 Salmonella cells can already pose a severe risk (University of Georgia, 2015).
Natural alternatives to antibiotics: effective Salmonella control?
To reduce the incidence of Salmonella while simultaneously lowering the use of antibiotics in animal production, there are different possibilities. On the one hand, veterinary medicine offers vaccines. On the other hand, the feed industry provides additives that strengthen the immune system, improve gut health, or support the animals in another manner. Other than pro- and prebiotics, the main active ingredient categories for such additives are organic acids and phytomolecules.
Organic acids worsen the conditions for Salmonella
Already in ancient Egypt, the method of fermentation and the generated acids have been used for the conservation of food (Ohmomo et al., 2002). Nowadays, it is a standard tool to protect feed (silage) and food from spoilage. Also for animals, organic acids added to the feed or the water have proven helpful against pathogens. These modes of action can be combined against Salmonella: reducing the pathogen load in the feed to limit the intake of bacteria and fighting against these pathogens in the animal.
Organic acids reduce Salmonella in feed materials
In general, the antimicrobial activity of organic acids in feed is based on lowering the pH (Pearlin et al., 2019). pH-sensitive bacteria such as Salmonella minimize their proliferation at a pH <5. Additionally, the organic acids attack bacteria directly. The acid’s undissociated and more lipophilic form penetrates the bacterial cell membrane. At the neutral pH within the cell, the acid dissociates, releases protons, and lowers the pH, leading to the impediment of metabolic processes in the cell. The cell spends a lot of energy trying to get the pH back to neutral (Mroz et al., 2006). Additionally, the anions become toxic for the cell metabolites and disrupt the membrane (Russel, 1992).
What do organic acids do in the bird?
According to Hernández and co-workers (2006) and Thompson and Hinton (1997), the addition of organic acids to the feed does not change the pH in the various digestive tract segments. Still, literature shows a clear reduction of Salmonella in the gut or litter when using propionic or/and formic acid (McHan and Emmett, 1992; Hinton and Linton, 1988; Humphrey & Lanning, 1988). A likely mode of action is described by Van Immerseel et al. (2004). He asserts that SCFAs such as propionic and formic acid as well as MCFAs can inhibit Salmonella’s penetration of the intestinal epithelium and, therefore, can control these invasive phenotypes of Salmonella (S. Typhimurium and S. Enteritidis).
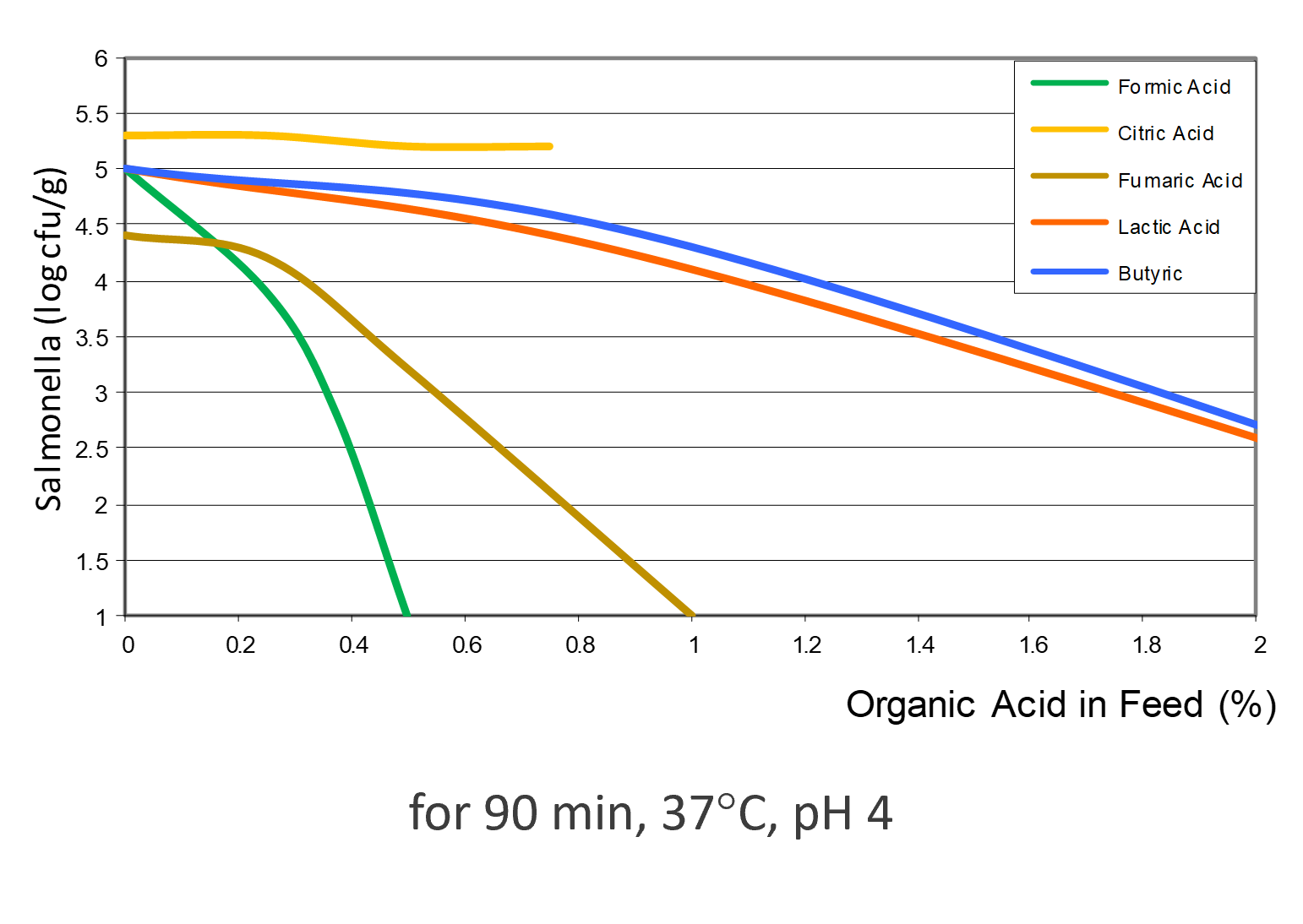
Different acids show different efficacy
Depending on the acid, the efficacy against Salmonella varies (see figure 3). Formic acid shows the highest effect, followed by fumaric acid. Then, lactic, butyric, and citric acid follow, showing lower efficacy.
Trials prove the efficacy of organic acids
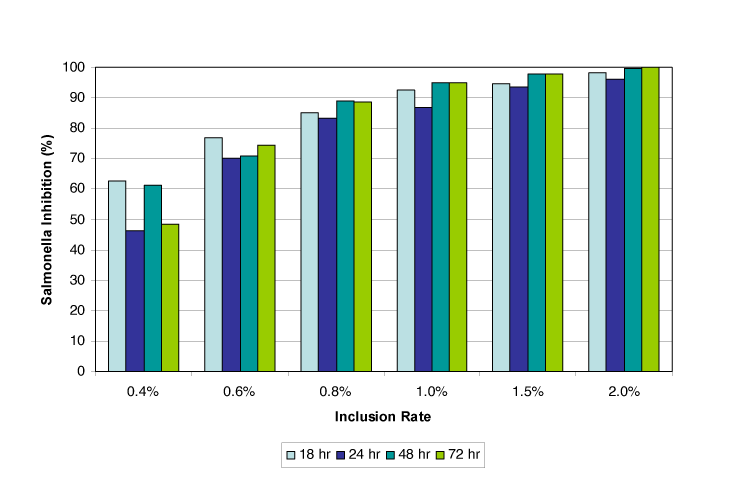
An in-vitro trial was conducted at a commercial research facility in the US to test the efficacy of Acidomix AFL, a liquid mixture of propionic and formic acid, against Salmonella. The bacterial strain used in these studies was nalidixic acid-resistant Salmonella typhimurium. The bacteria were maintained in broth cultures of tryptic soy broth.
They were added to 5 g of dry feed in a 50 ml tube to a final concentration of 40,000 CFU/g. Next, Acidomix AFL was added to the desired inclusion rate, and the samples were incubated at room temperature. After 18 to 72 hours of incubation, viable bacteria were counted using the plate count method.
Results: As shown in figure 4, the trial found that at an inclusion rate of 2.0 %, Salmonella inhibition was nearly 100 %. Already at a 0.4 % inclusion rate, Salmonella could be reduced by 45-60 %, showing a clear dose dependency.
Phytomolecules combat Salmonella through complex modes of action
Plants produce phytogenic substances to protect themselves from molds, yeasts, and bacteria, among others. After several purification steps, these phytomolecules can be used to fight Salmonella in poultry. They work through different modes of action, from attacking the cell wall (terpenoids and phenols) to influencing the genetic material of the pathogenic cells or changing the whole morphology of the cell.
Due to the different modes of action, it was long thought that there would be no resistance development. Still, Khan et al. (2009) found some microorganisms such as multidrug-resistant E. coli, Klebsiella pneumoniae, S. aureus, Enterococcus faecalis, Pseudomonas aeruginosa, and Salmonella typhimurium can show a certain – perhaps natural – resistance to some components of herbal medicines.
Gram-negative bacteria such as Salmonella are usually less attackable by phytomolecules because the cell wall only allows small hydrophilic solutes to pass; however, phytomolecules are hydrophobic. However, mixing the phytomolecules with an emulsifier facilitates the invasion into the cell. Their efficacy depends on their chemical composition. It is also decisive if single substances or blends (possible positive or negative synergies) are used.
The best-clarified mode of action is the one of thymol and carvacrol, the major components of the oils of thyme and oregano. They can get into the bacterial membrane and disrupt its integrity. The permeability of the cell membrane for ions and other small molecules such as ATP increases, decreasing the electrochemical gradient above the cell membrane and the loss of energy equivalents.
Trials show the efficacy of phytomolecules against Salmonella
Two different phytogenic compositions were tested for their efficacy against Salmonella.
Trial 1: Blend of phytomolecules and organic acids shows best results in an in-vitro assay
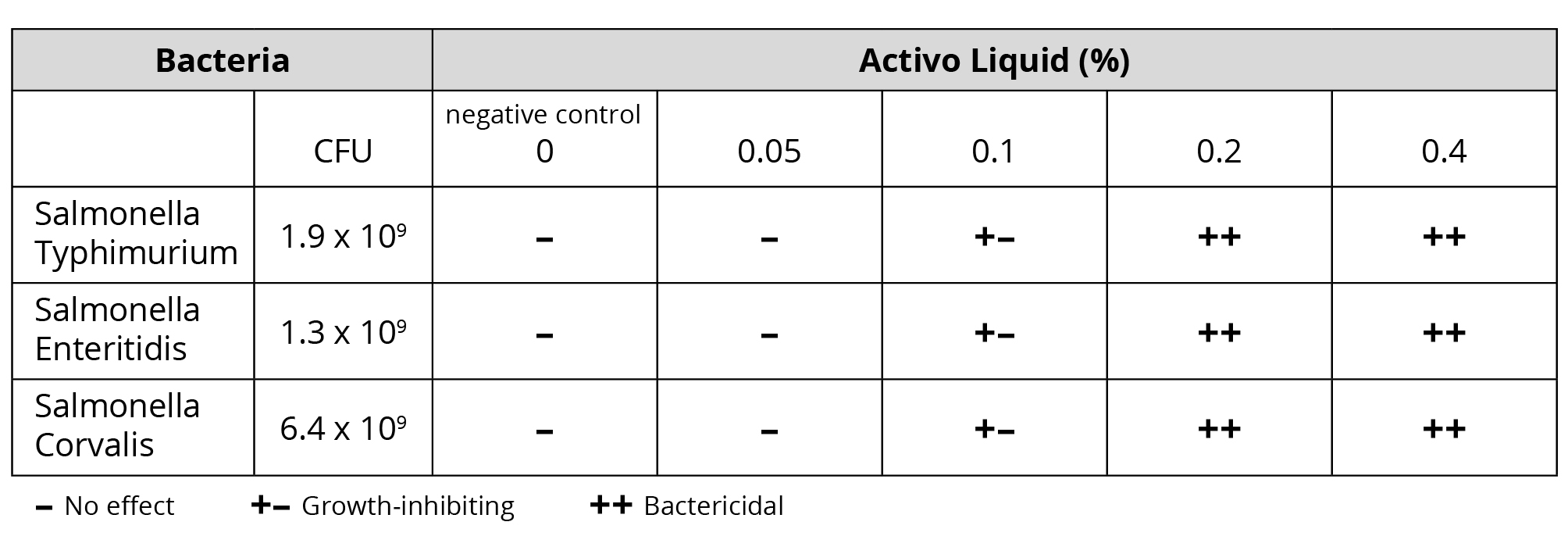
To evaluate its potential as a tool for antibiotic reduction, a trial was conducted to test the antimicrobial properties of Activo Liquid, a mixture of selected phytomolecules and an organic acid designed for application in water. The laboratory test was carried out at the Veterinary Diagnosis Department of Kasetsart University in Thailand. Standardized suspensions [1×104 CFU/ml] of three poultry-relevant Salmonella strains were incubated in LB medium, either without or with Activo Liquid. The tests were run at concentrations of 0.05%; 0.1%; 0.2% and 0.4%. After incubation at 37°C for 6-7 hours, serial dilutions of the cell suspensions were transferred onto LB agar plates and incubated for 18-22h at 37°C. Subsequently, colonies (CFU/ml) were determined.
Results: Activo Liquid was found to be growth-inhibiting to all Salmonella strains from a concentration of 0.1% onwards. At 0.2%, Activo Liquid already exhibited bactericidal efficacy against all tested Salmonella isolates, which was confirmed at a concentration of 0.4%.
Trial 2: Blend of nature-identical phytomolecules inhibits Salmonella
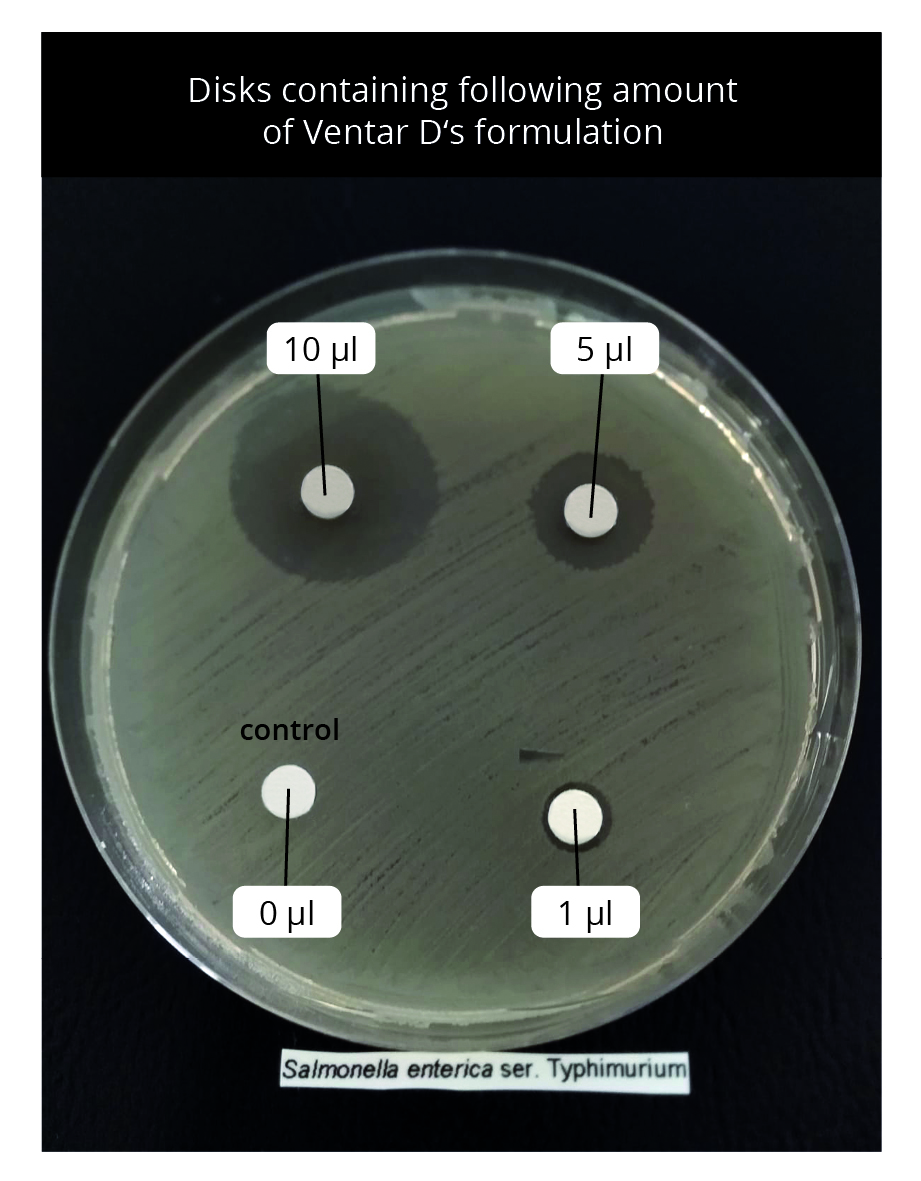
On Mueller Hinton agar plates where Salmonella enterica were spread uniformly, small disks containing 0 (control, only methanol), 1, 5, and 10 µl of Ventar D were placed and incubated at 37 °C for 16 hours. The presence of clearing zones indicates antimicrobial activity.
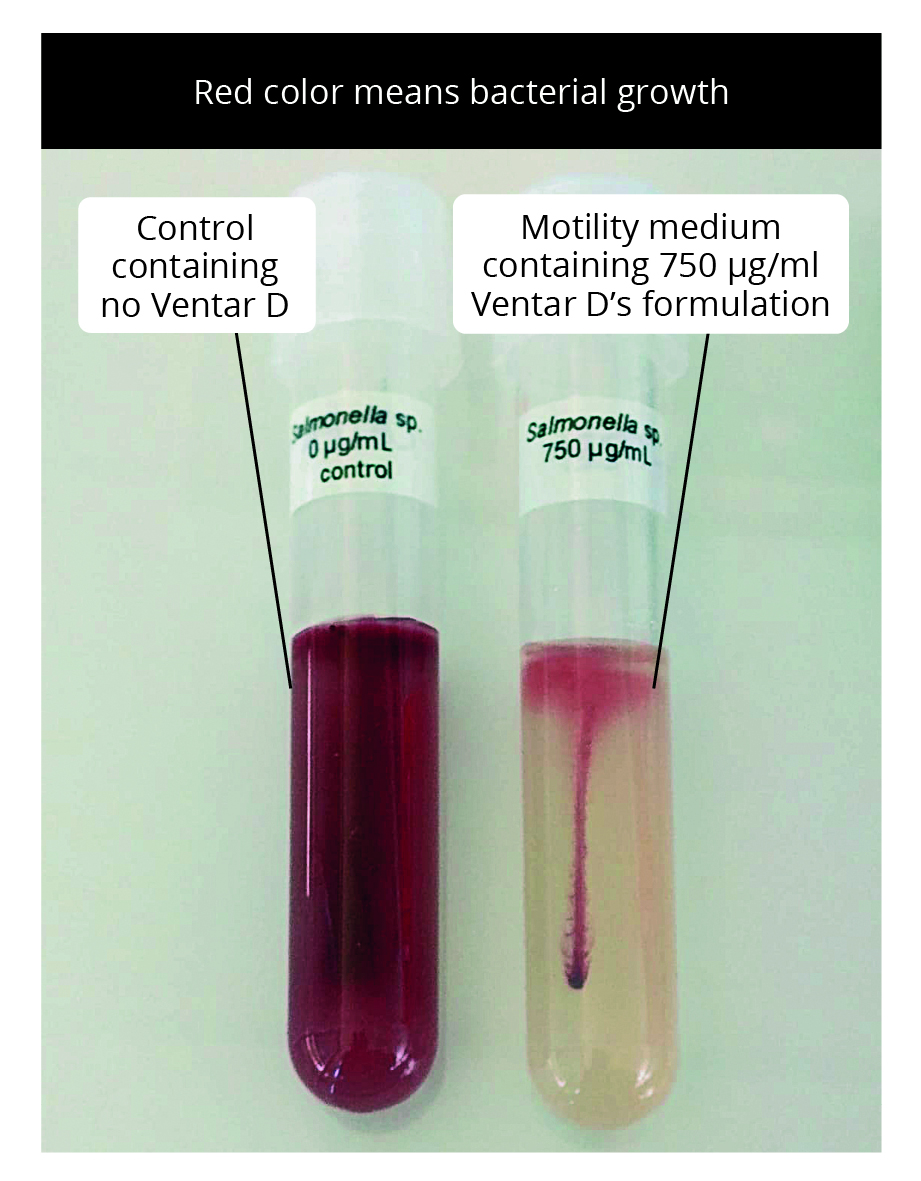
Additionally, a motility test was performed in tubes with a motility test medium containing 0 (control) and 750 µL Ventar D. For this purpose, one colony of Salmonella enterica grown on the agar was stuck in the middle of the medium and incubated at 37 °C for 12-16 hours. Growth can be visualized through the formation of red color.
Result: Ventar D inhibited S. enterica in a dose-dependent manner. Clearing zones were visible within the lowest tested concentration. At its inhibitory concentration, Ventar D suppressed S. enterica motility (figures 5 and 6).
Let’s fight Salmonella through effective and sustainable natural tools
The zoonosis Salmonella generates high costs in the poultry industry. As Salmonellosis can be transferred to humans, it must be kept under control by all means. Antibiotics are one tool to fight Salmonella, but they have their “side effects”: they are no longer well respected by the consumer, and, even more critically, they create resistance. To help keep antibiotics effective, poultry producers seek to use effective but not resistance-creating natural solutions against Salmonella.
As shown with the reviewed trials, organic acids and phytomolecules are highly active against diverse Salmonella serovars. Accordingly, feed additives based on these active ingredients offer effective tools for controlling Salmonella in poultry while also contributing to the overarching aim of reducing antibiotic use in poultry production.
References
Bell, Chris, and Alec Kyriakides. Salmonella: A Practical Approach to the Organism and Its Control in Foods. Oxford: Blackwell Science, 2002.
Castro-Vargas, Rafael Enrique, María Paula Herrera-Sánchez, Roy Rodríguez-Hernández, and Iang Schroniltgen Rondón-Barragán. “Antibiotic Resistance in Salmonella Spp. Isolated from Poultry: A Global Overview.” October-2020 13, no. 10 (October 3, 2020): 2070–84. https://doi.org/10.14202/vetworld.2020.2070-2084.
CDC. “Serotypes and the Importance of Serotyping Salmonella.” Centers for Disease Control and Prevention, February 21, 2020. https://www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotyping-importance.html.
EFSA. “Salmonella.” European Food Safety Authority. Accessed February 1, 2022. https://www.efsa.europa.eu/en/topics/topic/salmonella.
Ehuwa, Olugbenga, Amit K. Jaiswal, and Swarna Jaiswal. “Salmonella, Food Safety and Food Handling Practices.” Foods 10, no. 5 (2021): 907. https://doi.org/10.3390/foods10050907.
FDA. “Get the Facts about Salmonella.” U.S. Food and Drug Administration, July 28, 2020. https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-about-salmonella.
Fischer, Andreas. “Clever Infiziert – Die Tricks Der Bakterien.” HZI – Helmholtz Zentrum für Infektionsforschung, August 19, 2021. https://www.helmholtz-hzi.de/de/aktuelles/thema/clever-infiziert-die-tricks-der-bakterien/.
Forsythe, Steve J. The Microbiology of Safe Food. Hoboken, NJ: Wiley-Blackwell, 2020.
Gheisari, A.A., M. Heidari, R.K. Kermanshahi, M. Togani, and S. Saraeian. “Effect of Dietary Supplementation of Protected Organic …” WPSA, 2007. https://www.cabi.org/Uploads/animal-science/worlds-poultry-science-association/WPSA-france-2007/74.pdf.
Guillén, Silvia, María Marcén, Ignacio Álvarez, Pilar Mañas, and Guillermo Cebrián. “Stress Resistance of Emerging Poultry-Associated Salmonella Serovars.” International Journal of Food Microbiology 335 (2020): 108884. https://doi.org/10.1016/j.ijfoodmicro.2020.108884.
Hernández, F., V. García, J. Madrid, J. Orengo, P. Catalá, and M.D. Megías. “Effect of Formic Acid on Performance, Digestibility, Intestinal Histomorphology and Plasma Metabolite Levels of Broiler Chickens.” British Poultry Science 47, no. 1 (2006): 50–56. https://doi.org/10.1080/00071660500475574.
Hinton, M. “Antibacterial Activity of Short-Chain Fatty Acids.” The Veterinary Record 126 (n.d.): 416–21.
Hinton, M., and A. Linton. “Control of Salmonella Infections in Broiler Chickens by the Acid Treatment of Their Feed.” Veterinary Record 123, no. 16 (1988): 416–21. https://doi.org/10.1136/vr.123.16.416.
Humphrey, T. J., and D. G. Lanning. “The Vertical Transmission of Salmonellas and Formic Acid Treatment of Chicken Feed: A Possible Strategy for Control.” Epidemiology and Infection 100, no. 1 (1988): 43–49. https://doi.org/10.1017/s0950268800065547.
Khan, Rosina, Barira Islam, Mohd Akram, Shazi Shakil, Anis Ahmad Ahmad, S. Manazir Ali, Mashiatullah Siddiqui, and Asad Khan. “Antimicrobial Activity of Five Herbal Extracts against Multi Drug Resistant (MDR) Strains of Bacteria and Fungus of Clinical Origin.” Molecules 14, no. 2 (2009): 586–97. https://doi.org/10.3390/molecules14020586.
Koutsoumanis, Kostas, Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Alessandra De Cesare, et al. “Salmonella Control in Poultry Flocks and Its Public Health Impact.” EFSA Journal 17, no. 2 (2019). https://doi.org/10.2903/j.efsa.2019.5596.
Maciel, Bianca Mendes, Rachel Passos Rezende, and Nammalwar Sriranganathan. “Salmonella Enterica: Latency.” Current Topics in Salmonella and Salmonellosis, 2017. https://doi.org/10.5772/67173.
McHan, Frank, and Emmett B. Shotts. “Effect of Feeding Selected Short-Chain Fatty Acids on the in Vivo Attachment of Salmonella Typhimurium in Chick Ceca.” Avian Diseases 36, no. 1 (1992): 139. https://doi.org/10.2307/1591728.
Mkangara, M. and M., R. Mwakapuja, J. Chilongola, P. Ndakidemi, E. Mbega, and M. Chacha. “Mechanisms for Salmonella Infection and Potential Management Options in Chicken.” The Journal of Animal & Plant Sciences 30, no. 2 (April 2, 2020): 259–79. https://doi.org/10.36899/japs.2020.2.0050.
Mroz, Z., S.-J. Koopmans, A. Bannink, K. Partanen, W. Krasucki, M. Øverland, and S. Radcliffe. “Chapter 4 Carboxylic Acids as Bioregulators and Gut Growth Promoters in Nonruminants.” Biology of Growing Animals, 2006, 81–133. https://doi.org/10.1016/s1877-1823(09)70091-8.
OHMOMO, Sadahiro, Osamu TANAKA, Hiroko K. KITAMOTO, and Yimin CAI. “Silage and Microbial Performance, Old Story but New Problems.” Japan Agricultural Research Quarterly: JARQ 36, no. 2 (2002): 59–71. https://doi.org/10.6090/jarq.36.59.
Ranieri, Matthew L., Chunlei Shi, Andrea I. Moreno Switt, Henk C. den Bakker, and Martin Wiedmann. “Comparison of Typing Methods with a New Procedure Based on Sequence Characterization for Salmonella Serovar Prediction.” Journal of Clinical Microbiology 51, no. 6 (2013): 1786–97. https://doi.org/10.1128/jcm.03201-12.
Russell, J.B. “Another Explanation for the Toxicity of Fermentation Acids at Low Ph: Anion Accumulation versus Uncoupling.” Journal of Applied Bacteriology 73, no. 5 (1992): 363–70. https://doi.org/10.1111/j.1365-2672.1992.tb04990.x.
Thompson, J. L., and M. Hinton. “Antibacterial Activity of Formic and Propionic Acids in the Diet of Hens on Salmonellas in the Crop.” British Poultry Science 38, no. 1 (1997): 59–65. https://doi.org/10.1080/00071669708417941.
USDA – United States Department of Agriculture – Research, Education & Economics Information System. University of Georgia, 2015. https://portal.nifa.usda.gov/web/crisprojectpages/0228031-effect-of-fat-content-on-the-survival-of-salmonella-in-food.html.
“USDA Launches New Effort to Reduce Salmonella Illnesses Linked to Poultry.” USDA, October 19, 2021. https://www.usda.gov/media/press-releases/2021/10/19/usda-launches-new-effort-reduce-salmonella-illnesses-linked-poultry.
Van Immerseel, F., J. B. Russell, M. D. Flythe, I. Gantois, L. Timbermont, F. Pasmans, F. Haesebrouck, and R. Ducatelle. “The Use of Organic Acids to Combatsalmonellain Poultry: A Mechanistic Explanation of the Efficacy.” Avian Pathology 35, no. 3 (2006): 182–88. https://doi.org/10.1080/03079450600711045.
Van Immerseel, Filip, Jeroen De Buck, Isabel De Smet, Frank Pasmans, Freddy Haesebrouck, and Richard Ducatelle. “Interactions of Butyric Acid– and Acetic Acid–Treated Salmonella with Chicken Primary Cecal Epithelial Cells in Vitro.” Avian Diseases 48, no. 2 (2004): 384–91. https://doi.org/10.1637/7094.
Visscher, C. “Über Das Futter Helfen – Den Salmonellen Das Leben Schwer Machen.” Bauernblatt Schleswig-Holstein + Hamburg 68/164, no. 51 (December 20, 2014): 66–68.
Wernicki, Andrzej, Anna Nowaczek, and Renata Urban-Chmiel. “Bacteriophage Therapy to Combat Bacterial Infections in Poultry.” Virology Journal 14, no. 1 (September 16, 2017). https://doi.org/10.1186/s12985-017-0849-7.
Wessels, Kirsten, Diane Rip, and Pieter Gouws. “Salmonella in Chicken Meat: Consumption, Outbreaks, Characteristics, Current Control Methods and the Potential of Bacteriophage Use.” Foods 10, no. 8 (2021): 1742. https://doi.org/10.3390/foods10081742.