We all know the headlines, “European Commission adopts ZnO ban” or “Zinc oxide to be phased out at EU level by 2022”. Clearly, EU legislation has far-reaching consequences for European pig producers – but in the jungle of acronyms and legalistic jargon, it’s not always clear which institution gets to decide what and why. Here are five key facts that help pig producers make sense of the EU’s zinc oxide ban.
1. Zinc oxide can only be used as a feed additive (low dosage)
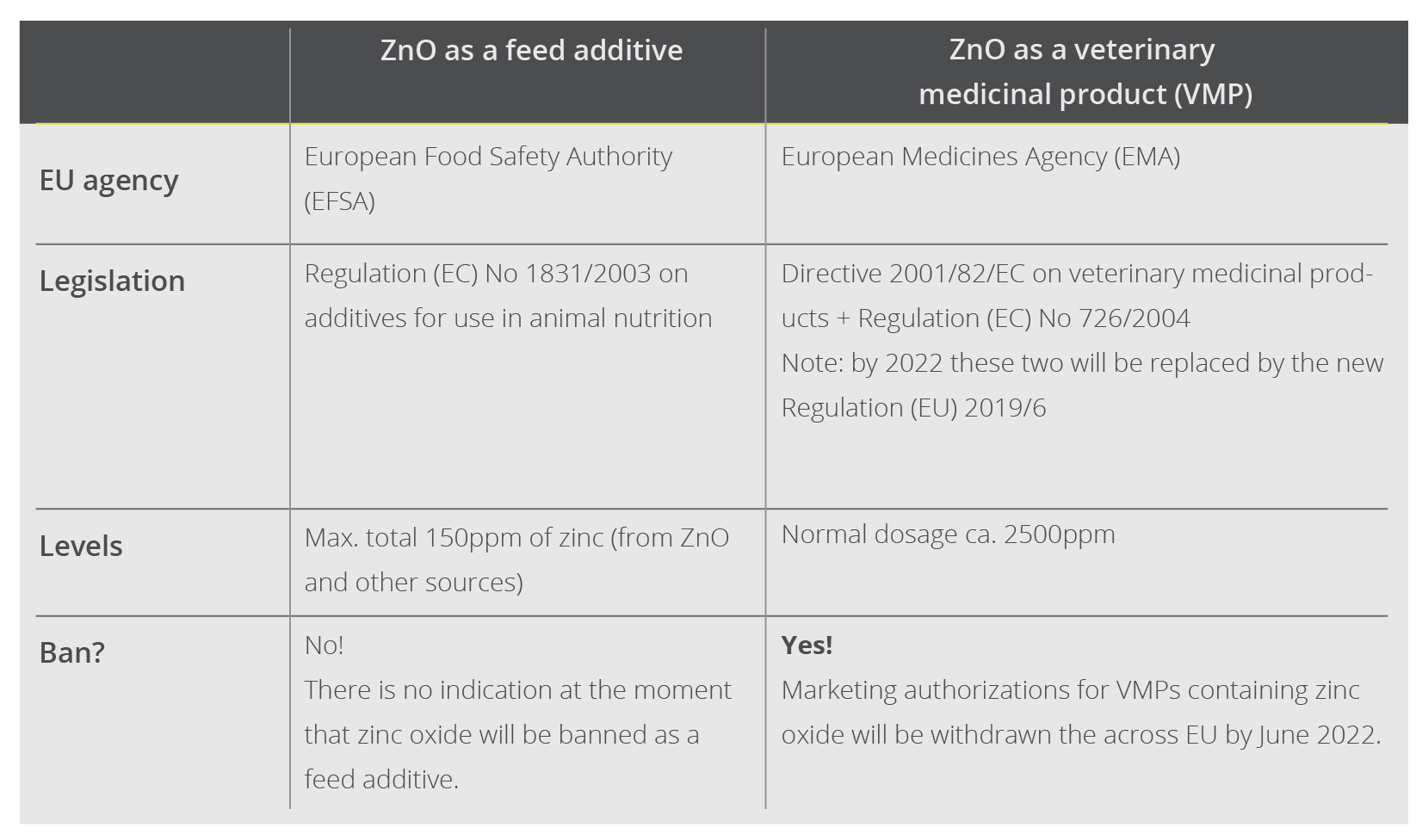
Pigs require zinc to maintain various metabolic functions, hence it is included in their diet as a feed additive. This use will not be banned: ZnO is included as a source of zinc in the so-called register of feed additives, which applies to the whole EU. The European Commission decides which products are included in the register based on the opinions of the European Food Safety Authority (EFSA), which also advises the Commission on topics like animal welfare and African swine fever. The EFSA currently suggests that a total level of 150ppm meets the animals’ physiological needs for zinc. The European Commission has turned this recommendation into law, hence 150ppm is the legal limit for zinc supplementation for piglets.
2. The EU sets common rules for veterinary medicinal products
ZnO-based products to treat post-weaning diarrhea in piglets, on the other hand, contain pharmacological doses of zinc oxide. A commonly administered dosage is 100mg per kg body weight per day for 14 consecutive days, amounting to 2500ppm zinc in the feed. These products are classified as veterinary medicinal products (VMPs) and are thus covered by Directive 2001/82/EC on medicinal products for veterinary use and by Regulation (EC) No 726/2004. These pieces of legislation set out the EU’s rules for the production, distribution, and authorizations of VMPs, and they establish the European Medicines Agency (EMA). Just as the EFSA advises the European Commission on feed additives, they turn to the EMA regarding VMPs.

Zinc oxide – two different uses, two different situations
3. ZnO products licenses are a national topic – but subject to EU scrutiny
One of EMA’s key topics are marketing authorizations: VMPs can only be sold and traded in the EU if they have received a marketing authorization, which is basically a license. Depending on the type of VMP and on when it was first released, the marketing authorization is either issued by the EMA or by national authorities. Veterinary medicines containing zinc oxide are (or rather were) within the remit of national authorization procedures. However, national authorities are supposed to turn to the EMA’s Committee for Medicinal Products for Veterinary Use (CVMP) if they have any issues with an application that is submitted to them. This is what happened in the case of zinc oxide.
4. France and the Netherlands initiated the review of zinc oxide
A European company in the feed industry had applied for marketing authorization for its ZnO-based medicated feeding stuff for piglets in the United Kingdom, hoping for a so-called decentralized authorization procedure to take place. This procedure would mean that the marketing authorization issued in the UK would also be valid in other EU countries. However, France and the Netherlands objected to this on the grounds of environmental concerns. Initially, the CVMP ruled that the marketing authorization could be granted, but France and the Netherlands persisted. In a second round, they raised doubts about the efficacy of risk mitigation measures and the added issue of antimicrobial resistance. This time, they were successful.
5. Bottom line: ZnO products will no longer get a marketing authorization
In March 2017, the CVMP concluded that zinc oxide’s benefits of preventing diarrhea do not outweigh the risks to the environment. Therefore the panel recommended that national authorities withdraw existing marketing authorizations for zinc oxide-based VMPs and that they no longer grant new authorizations. Shortly after that, on 26 June 2017, the European Commission adopted the CVMP’s recommendation, which means that all EU countries have to implement it. This decision also says that countries may defer withdrawing the marketing authorizations if they think that the lack of available alternatives and necessary changes in farming practices put too much pressure on their pig sectors. They can only defer for five years though; hence, the decision must be implemented no later than 26 June 2022.
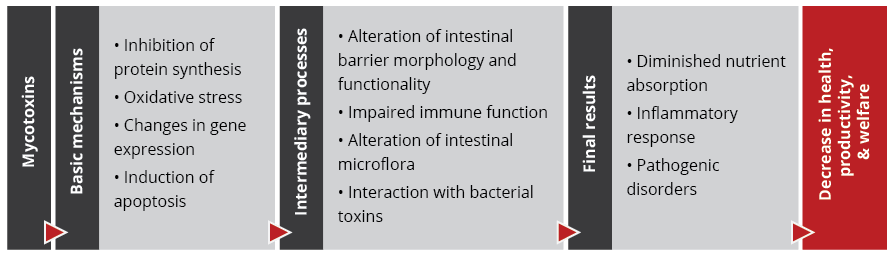 Today we stand at the half-way point before the ban of VMP ZnO as a veterinary medicinal product kicks in across the EU. Hence the search is on for effective strategies to control post-weaning diarrhea: without zinc but through continuous improvements in management and feed practices, as well as the support of targeted, functional feed additives.
Today we stand at the half-way point before the ban of VMP ZnO as a veterinary medicinal product kicks in across the EU. Hence the search is on for effective strategies to control post-weaning diarrhea: without zinc but through continuous improvements in management and feed practices, as well as the support of targeted, functional feed additives.
By Sabria Regragui Mazili – Content Editor EW Nutrition
Article available in german, dutch and spanish.