By Dr. Inge Heinzl, Editor, Madalina Diaconu, Produt Manager Pretect D, and Dr. Ajay Awati, Global Category Manager Gut Health & Nutrition, EW Nutrition
Often you have an extensive coccidiosis control program in place. You don’t observe any clinical signs of coccidiosis. However, at the end of the cycle, you record significantly lower body weight and a higher FCR. There is a high probability that your animals have subclinical coccidiosis. This article digs deeper into understanding why birds don’t perform as they should, why subclinical coccidiosis occurs on the farm, and why drug resistance is an important factor.
Subclinical coccidiosis – a silent enemy
Clinical coccidiosis is clearly characterized by severe diarrhea, high mortality rates, reduced feed/water intake, and weight loss. By contrast, subclinical Coccidiosis does not display any visual signs and often remains undetected.
According to De Gussem (2008), the damages caused by subclinical coccidiosis can reach up to 70% of the total cost of coccidiosis control treatments, ranging from US$ 2.3 billion to US$ 13.8 billion/year in 2020 worldwide (De Gussem, 2008; Ferreira da Cunha, 2020; Blake et al., 2020).
Monitoring coccidiosis occurrence on the farm
There are several tools available to evaluate the level of infection. The most common ones are:
Lesion scoring – is used to evaluate the damages caused by coccidiosis in the intestinal tract. Lesion scoring gives insight into the severity of the infection. Furthermore, based on the location of lesions in the GI tract, it is possible to determine the plausible Eimeria spp. responsible for the infection.
OPG (Oocyst per gram) – the number of oocysts per gram of feces indicates the level of shedding of oocysts in the manure, litter, and, eventually, in the farm environment. OPG levels may not give the exact severity of the infection in the bird but certainly provide a clear idea of its likely spread within the flock.
Ways to deal with coccidiosis on the farm
Different tools are widely used to prevent and treat coccidiosis:
Anticoccidials: Chemicals, ionophores
Vaccination: Natural strains, attenuated strains
Bio-shuttle: Vaccine + ionophore
Natural anticoccidials: Phytomolecules
These coccidiosis control programs are used depending on the farm history and the severity of the infection. Traditionally, treatment was heavily dependent on chemicals and ionophores. However, rampant and unbridled use of ionophores leads to resistance in Eimeria spp. on the farm, the failure of the control program, and significant performance losses, with high mortality due to coccidiosis. Therefore, the tools mentioned above are inserted in rotation or shuttle programs to minimize the generation of resistances. In a rotation program, the anticoccidial changes from flock to flock. In a shuttle program, the anticoccidial changes within one cycle according to the feed (Chapman, 1997).
However, this strategy is often not 100% effective due to a lack of diversity and overuse of certain tools within programs. The rigorous financial optimization of the program leads to the use of cost-effective but marginally effective solutions. These factors over the period weaken the program, which seems to work well but leads to resistance to anticoccidial drugs and sets up subclinical coccidiosis.
Resistances have been reported in the US (Jeffers, 1974, McDougald, 1981), South America (McDougald, 1987; Kawazoe and Di Fabio, 1994), Europe (Peeters et al., 1994; Bedrník et al., 1989; Stephan et al., 1997), Asia (Lan et al., 2017; Arabkhazaeli et al., 2013), and Africa (Ojimelukwe et al., 2018). Chapman and co-workers (1997) even stated that resistances were documented for all anticoccidial drugs employed at this time, and new products have not been approved for decades.
Resistance and subclinical coccidiosis can be approached naturally
When an anticoccidial has lost its effectiveness due to excessive use, some resistant coccidia survive. They can cause a mild course of the disease, subclinical coccidiosis, driving the costs high. Reducing the occurrence of resistance and subclinical coccidiosis can significantly decrease the expenses of coccidiosis control programs and, eventually, the cost of production.
Increasing consumer pressure to reduce the overall usage of drugs in animal production has driven innovation efforts to find natural solutions that can be effectively used within coccidiosis control programs. However, this shift was not easy for the producers. Lack of reliable data, poor understanding of the mode of action, lack of quality optimization, and unsubstantiated claims led to the failure of many earlier-generation natural solutions.
However, the consumer-driven movement to find natural solutions to animal gut health issues has recently led to relentless innovation in this area. Knowledge, research, and technological developments are now ready to offer solutions that can be an effective part of the coccidia control program and open opportunities to make poultry production even more sustainable by reducing drug dependency.
For centuries, phytomolecules have been used for their medicinal properties and effects on the health and well-being of animals and humans. In the case of coccidiosis, tannins and saponins have been proven to support animals in coping with this disease. Tannic acids and tannic acid extracts strengthen the intestinal barrier by reducing oxidative stress and inflammation (Tonda et al., 2018). On the other hand, saponins lessen the shedding of oocysts, improve the lesion score, and, in the case of an acute infection, the occurrence of bloody diarrhea (Youssef et al., 2021).
These natural substances can be integrated into shuttle or rotation programs to reduce the use of anticoccidials and, therefore, minimize resistance development.
Pretect D: Coccidiosis programs can be strengthened naturally!
In an EU field trial conducted with more than 200 000 birds, Pretect D (a natural phytogenic-based product designed to increase the efficacy of coccidiosis control) was used in the shuttle program together with ionophores. The trial provided excellent results on zootechnical performance (figures 1-4).
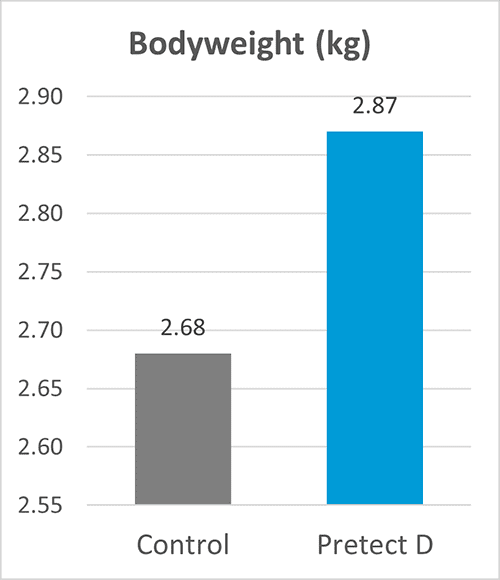
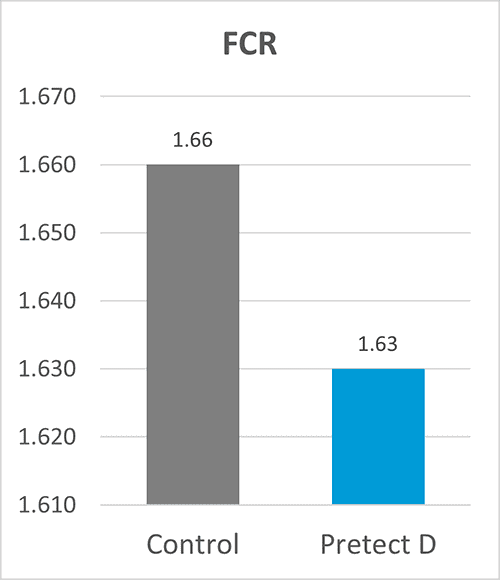
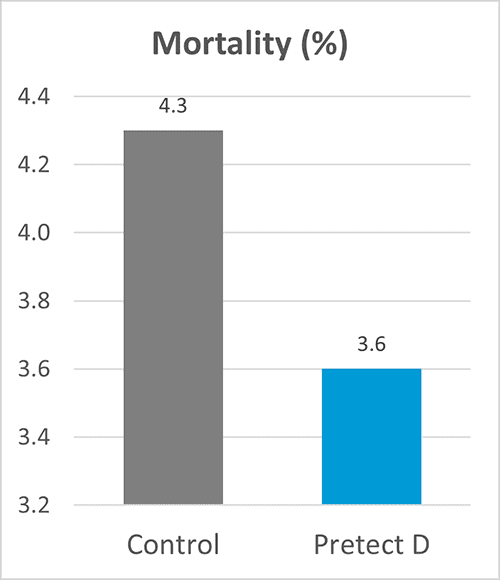
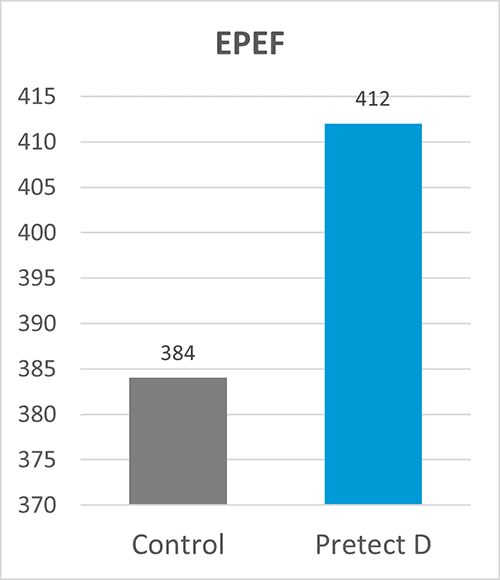
Figures 1-4: Zootechnical performance of broilers with Pretect D included in the shuttle program
Trials show that Pretect D supports the efficiency of coccidiosis control programs by impairing the Eimeria development cycle when used in combination with vaccines, ionophores, and chemicals as part of the shuttle or rotation program:
- It protects the epithelium from inflammatory and oxidative damage
- It promotes the restoration of the mucosal barrier function
Table 1 exemplifies one way of including a natural solution (Pretect D) in actual coccidiosis control programs.
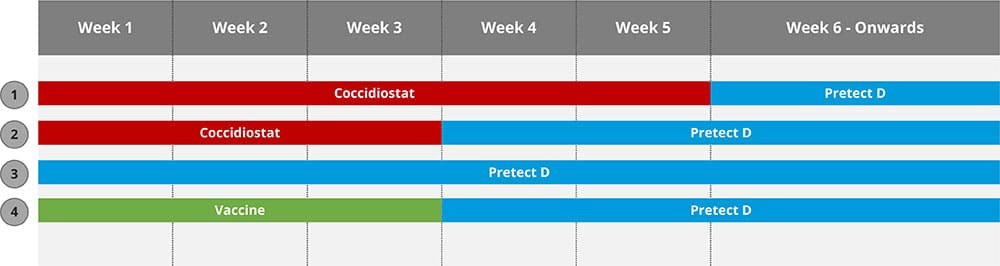 Table 1: Exemple of including Pretect D into coccidiosis control programs
Table 1: Exemple of including Pretect D into coccidiosis control programs
Natural solutions suit both farmers and consumers
With phytomolecules partly replacing anticoccidials in rotation or shuttle programs, the use of anticoccidials in poultry production can be decreased. On the one hand, this answers consumers’ demand; on the other hand, it leads to a push-back of resistances in the long run. The returning effectiveness of the anticoccidials can reduce subclinical coccidiosis, leading to lower costs spent on this disease and a higher profit for the farmers.
References:
Arabkhazaeli, F., M. Modrisanei, S. Nabian, B. Mansoori, and A. Madani. “Evaluating the Resistance of Eimeria spp. Field Isolates to Anticoccidial Drugs Using Three Different Indices.” Iran J Parasitol. 8, no. 2 (2013): 234–41.
Bedrník, P., P. Jurkovič, J. Kučera, and A. Firmanová. “Cross Resistance to the IONOPHOROUS Polyether Anticoccidial Drugs IN Eimeria Tenella Isolates from Czechoslovakia.” Poultry Science 68, no. 1 (1989): 89–93. https://doi.org/10.3382/ps.0680089
Blake, Damer P., Jolene Knox, Ben Dehaeck, Ben Huntington, Thilak Rathinam, Venu Ravipati, Simeon Ayoade, et al. “Re-Calculating the Cost of Coccidiosis in Chickens.” Veterinary Research 51, no. 1 (2020). https://doi.org/10.1186/s13567-020-00837-2
Chapman, H. D. “Biochemical, Genetic and Applied Aspects of Drug Resistance in Eimeria Parasites of the Fowl.” Avian Pathology 26, no. 2 (1997): 221–44. https://doi.org/10.1080/03079459708419208.
De Gussem, M., and S. Huang. “The Control of Coccidiosis in Poultry.” International Poultry Production 16, no. 5 (2008): 7–9.
Ferreira da Cunha, Anderson, Elizabeth Santin, and Michael Kogut. “Editorial: Poultry Coccidiosis: Strategies to Understand and Control.” Frontiers in Veterinary Science 7 (2020). https://doi.org/10.3389/fvets.2020.599322
Jeffers, T. K. “Eimeria Acervulina and E. Maxima: Incidence and Anticoccidial Drug Resistance of Isolants in Major Broiler-Producing Areas.” Avian Diseases 18, no. 3 (1974): 331. https://doi.org/10.2307/1589101
Kawazoe, Urara, and J. Di Fabio. “Resistance to DICLAZURIL in Field Isolates OfEimeriaspecies Obtained from Commercial BROILER Flocks in Brazil.” Avian Pathology 23, no. 2 (1994): 305–11. https://doi.org/10.1080/03079459408418998
Lan, L.-H., B.-B. Sun, B.-X.-Z. Zuo, X.-Q. Chen, and A.-F. Du. “Prevalence and Drug Resistance of Avian Eimeria Species in Broiler Chicken Farms of Zhejiang PROVINCE, CHINA.” Poultry Science 96, no. 7 (2017): 2104–9. https://doi.org/10.3382/ps/pew499
McDougald, L. R. “Anticoccidial Drug Resistance in the Southeastern United STATES: POLYETHER, IONOPHOROUS Drugs.” Avian Diseases 25, no. 3 (1981): 600. https://doi.org/10.2307/1589990
McDougald, Larry R., Jose Maria Silva, Juan Solis, and Mauricio Braga. “A Survey of Sensitivity to Anticoccidial Drugs in 60 Isolates of Coccidia from Broiler Chickens in Brazil and Argentina.” Avian Diseases 31, no. 2 (1987): 287. https://doi.org/10.2307/1590874
Ojimelukwe, Agatha E., Deborah E. Emedhem, Gabriel O. Agu, Florence O. Nduka, and Austin E. Abah. “Populations of Eimeria Tenella Express Resistance to Commonly Used Anticoccidial Drugs in Southern Nigeria.” International Journal of Veterinary Science and Medicine 6, no. 2 (2018): 192–200. https://doi.org/10.1016/j.ijvsm.2018.06.003
Peeters, Johan E., Jef Derijcke, Mark Verlinden, and Ria Wyffels. “Sensitivity of AVIAN EIMERIA Spp. to Seven Chemical and Five Ionophore Anticoccidials in Five Belgian INTEGRATED Broiler Operations.” Avian Diseases 38, no. 3 (1994): 483. https://doi.org/10.2307/1592069
Stephan, B., M. Rommel, A. Daugschies, and A. Haberkorn. “Studies of Resistance to Anticoccidials IN Eimeria Field Isolates and Pure Eimeria Strains.” Veterinary Parasitology 69, no. 1-2 (1997): 19–29. https://doi.org/10.1016/s0304-4017(96)01096-5
Tonda, RM, J.K. Rubach, B.S. Lumpkins, G.F. Mathis, and M.J. Poss. “Effects of Tannic Acid Extract on Performance and Intestinal Health of Broiler Chickens Following Coccidiosis Vaccination and/or a Mixed-Species Eimeria Challenge.” Poultry Science 97, no. 9 (2018): 3031–42. https://doi.org/10.3382/ps/pey158
Youssef, Ibrahim M., Klaus Männer, and Jürgen Zentek. “Effect of Essential Oils or Saponins Alone or in Combination on Productive Performance, Intestinal Morphology and Digestive Enzymes’ Activity of Broiler Chickens.” Journal of Animal Physiology and Animal Nutrition 105, no. 1 (2020): 99–107. https://doi.org/10.1111/jpn.13431