Broiler production with reduced antibiotics. The essentials

By Dr. Inge Heinzl and Dr. Ajay Bhoyar – EW Nutrition
Concerns about antibiotic resistance in humans and production animals have prompted a push across the board to reduce antibiotic use, including in livestock rearing. To meet these demands, the industry must keep the pathogenic pressure in production units as low as possible, enabling production with no antibiotics or minimum use of antibiotics.

The 3 essential steps for reducing antibiotics in broiler production
In the following, we discuss experience-based insights and practical advice concerning best practices for broiler meat production with reduced antibiotic use, focusing on the following points:
- Farm biosecurity
- Management of the broiler house, including cleaning & disinfection, and environment & litter management
- Management of the flock, including DOC quality, disease prevention, and nutrition
1. General farm biosecurity
Biosecurity is the foundation for all disease prevention programs (Dewulf et al., 2018). Thus, it is essential in antibiotic reduction scenarios. It includes all measures taken to reduce the risk of introducing and spreading diseases, preventing diseases, and protecting against infectious agents. Its fundament is the knowledge of disease transmission processes.
The application of consistently high biosecurity standards substantially reduces antimicrobial resistance by preventing the introduction of resistance genes into the farm and lowering the need to use antimicrobials (Davies & DWales, 2019).
First of all: everyone must act in concert!
Biosecurity is one of the preconditions for the success of an ABR program, and it is crucial to bring all workers/staff on track through regular training on the best practices and their subsequent rigorous implementation. The biosecurity plan can only be effective if everyone on the operation follows it – all the time. Farm managers, poultry workers, and other persons entering the facility should adhere to the farm biosecurity measures, 24/24h – 7/7d.
Separation helps to prevent the spread of pathogens
One essential component for biosecurity is implementing a “line of separation” for the farm and each house. It is vital to have a good separation between high and low-risk animals and between areas on the farm that are dirty (general traffic) and clean (internal movements). In this way, it is not only possible to avoid the entrance but also the spread of disease, as potential sources of infection (e.g., wild birds) cannot reach the farm population.
The farm must be well isolated, not allowing the entry or passage of persons who do not work there and animals, including pets.
Inside the farm, the walls of the poultry house form the first line of separation, and the “Two-zone Danish Entry Protocol” constitutes a second line. This system utilizes a bench to divide the anteroom of a poultry house into two sides (outdoor / ‘dirty area’ and indoor / ‘clean area’). At a minimum, footwear should be changed, and hands washed or disinfected when passing over the bench; it is even better when workers have house-specific clothing and hairnets when entering the poultry area.
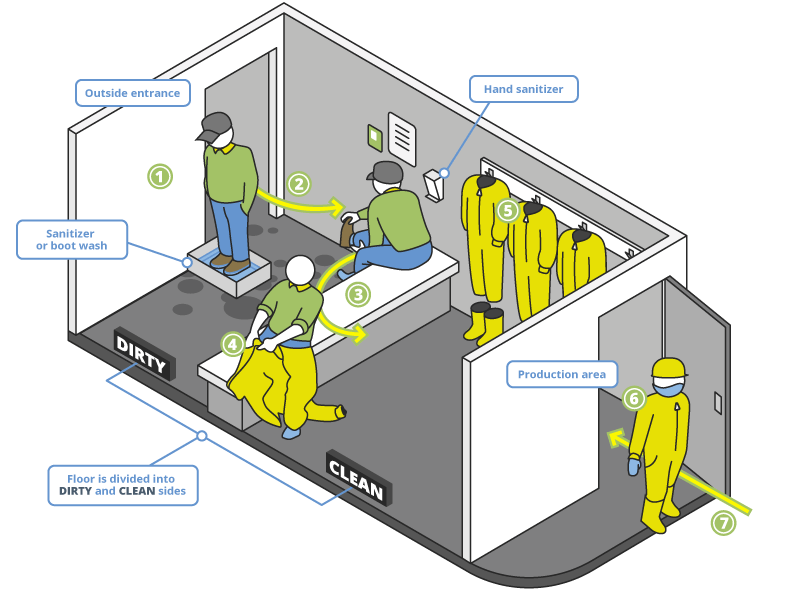
Figure 1: Safety procedures on the poultry farm – the Danish entry method
The room is divided into “dirty” and “clean” zones.
- After the entrance from outside, workers/visitors step into a disinfectant boot tray.
- They take off their street shoes and leave them on the dirty side of the entrance zone.
- Then, they turn from the dirty to the clean side by swinging their legs without touching the floor.
- They wash their hands and disinfect them by using the hand.
- They must put on an overall, cap, mask, and boots of the poultry house.
- Completely clothed, they can enter the poultry house.
- When they leave the house, a reversed process must be followed.
Still more needs to be done to prevent the entrance and spread of disease.
Separate materials for each house
For each house, separate materials must be used, keeping a dedicated set of tools and equipment necessary for daily work.
Very important: no materials should be moved from one house to another unless thoroughly disinfected. Crates for bird transport in the case of thinning (partial depopulation of a broiler flock) are an important example.
Practice clean disposal of mortality
First, dead birds’ removal must be frequent (minimum twice a day) as carcasses are a source of infection. The next point is to make sure the route of birds’ disposal is strictly unidirectional, and the buckets or wheelbarrows for the transport of the dead birds do not reenter the poultry house. Finally, the carcasses should remain outside the farm or as far from the buildings as possible until collection, incineration, or composting.
2. Broiler house management
After the general organization on the farm, let’s move on to the poultry houses.
Cleaning and disinfection of the house are the first steps – and check their efficacy!
Cleaning and disinfection are essential components in preventing the persistence and spread of pathogens. Both together aim to decrease microbial numbers on surfaces (and in the air) to a level that will ensure that most -if not all- pathogens and zoonotic agents are eliminated.
Cleaning refers to the physical removal of organic matter and biofilms, so the microorganisms and pathogens are afterward exposed to the disinfectant.
For effective cleaning and disinfection, the all-out/all-in system has proven of value. When birds are collected, all organic material, including feed residues and litter/feces, is removed.
Effective detergents and hot water are used to remove any grease or organic material. Pay special attention to the floors! Also, all surfaces and equipment should be sufficiently cleaned and given final disinfection.
Cleaning is crucial
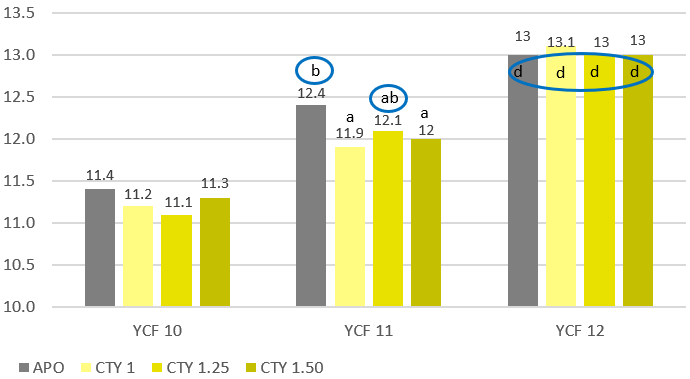
A study by Luyckx and collaborators (2015) revealed that the mean total aerobic bacterial count on swab samples taken in broiler houses decreases significantly already after cleaning (figure 2). Good cleaning not only strongly reduces microbiological contamination and organic material but also ensures that the subsequent disinfection has a stronger impact on the remaining microorganisms. Consider that all disinfectants, even in high concentrations, are barely effective in the presence of organic material.
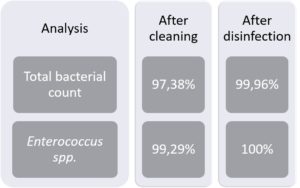
Figure 2: % of reduction of bacteria on surfaces after cleaning and after cleaning and disinfection (adapted from Luyckx et al., 2015)
Keep an eye on cleaning & disinfection efficacy
After cleaning and disinfection are complete, it is good practice to check the floors for Total Viable Count (TVC), Salmonella, and E. coli to test the efficacy of the cleaning and disinfection process. Recommended levels of TVC should be less than ten colony forming units per square centimeter (CFU/cm2), and E. coli and Salmonella levels should be undetectable.
When high TVC are found, the cleaning and disinfection procedure must be evaluated, including the products (a rotation is recommended) and their application (e.g., dosage, dilution, water temperature, and exposure time). Also, possible reinfection by vermin or personnel during the downtime must be controlled.
Downtime:After cleaning and disinfection, a down-time time of 10 days allows disease-causing pathogens to die (UC Davis, 2019). |
Cleaning and disinfection of the waterline against biofilm
In the waterlines, the build-up of biofilms can be an issue. Biofilm is a sticky film that can be found inside water lines, regulators, and nipple drinkers. It starts when bacteria attach to a surface and produce a matrix of extracellular polymeric substances (EPS), including proteins and sugars, giving the biofilm the stickiness that traps other bacteria and organic matter. It provides the bacteria with protection from the external environment, and thus they multiply and thrive.
Biofilms not only block the water flow, but they can also include pathogenic bacteria. Thus, the waterline must be regularly cleaned and disinfected, not only between flocks but also within each flock.

Between flocks, an effective waterline cleaning should include:
- Application of hydrogen peroxide at high concentration, leaving it in the system for 24-48 hours to remove the biofilm from the pipelines)
- Flush the line to remove the detached biofilm, also activate the nipples with a broom or stick to flush them
- Immediately before the placement of the new chicks, the water lines should be flushed to have fresh drinking water available to the chicks
- The water pressure must be adjusted so that a droplet of water is visible at the end of each nipple, and the drinkers are put to the correct height to stimulate water intake and avoid spilling
During the life of the birds, a water disinfectant should be used to prevent biofilm formation, e.g., hydrogen peroxide in weekly applications or the continuous use of chlorine. Also, flushing is a good practice during the whole cycle to make sure that biofilm is removed and the birds count with fresh drinking water.
To a certain extent, biofilm build-up can be prevented by using organic acidifiers in the water, which improves the sanitizers’ effectiveness and reduces bacterial growth in water lines.
Correct ventilation helps to prevent respiratory diseases
To keep broilers healthy, providing optimal ventilation in the poultry house is crucial. CO2 and temperature are the most critical parameters. CO2 should never exceed 2500 ppm and should be monitored continuously, most notably in the early morning before birds increase activity (e.g., eating). Ventilation rates should be adjusted to keep CO2 below this limit. Draught or cold spots resulting in uneven distribution of birds in the house should be avoided, and causes should be investigated and repaired immediately.
Incorrect ventilation often is the reason for respiratory diseases and the need for antibiotic treatment. No matter if natural or power ventilation is used, proper monitoring of the system is indispensable to ensure the well-functioning of the equipment and, therefore, appropriate air quality (Neetzon et al., 2017).
Litter management to keep diseases in check
Effective litter management is another step on the road to keeping the birds healthy. Dryness of litter and ammonia level at bird’s level are two significant key success factors in raising broilers. Dry litter preserves the footpads, so litter material should have a good moisture-absorbing capacity (e.g., chopped straw, wood shaving, rice husks, sunflower husks). When using build-up litter, litter sanitation and treatments need more attention.
Litter treatment (with acidifying or binding substances) and adequate ventilation are the most practical measures to control ammonia and improve littler quality (Malone, 2005). Keep litter temperature at 28 – 30°C (82.4 – 86°F), and use only litter tested or certified to have a TVC <10 CFU/g.
3. Flock management
The basis: healthy, high-quality day-old chicks
To produce good-quality day-old chicks, the parent flocks (PS) must be of good health status. PS should be free from vertically transmitted diseases such as Mycoplasma and Salmonella and be vaccinated/protected against important diseases:
- Salmonella pullorum/Salmonella Gallinari should be assessed in PS by RPA serology in week 25-30, at least 60 samples per flock.
- Mycoplasma gallisepticum should be checked by RPA/ELISA serology on a regular basis, preferably at least monthly, with a minimum of 30 samples per flock.
Parent flock vaccination leads to the production of maternal antibodies that help prevent horizontal infection (from the broiler farm environment) in chicks at an early age. This type of prevention is the primary function of some vaccinations, such as against Gumboro disease.
An essential part of the broilers’ life occurs already in the hatchery. Single-stage incubation is recommended, and all floor eggs and dirty nest eggs should be excluded to assure the best day-old chick quality.
Comfortable conditions bring chicks to eat
The brooding phase needs special attention; it is about welcoming the chicks and making them comfortable in the house environment. For this, enough litter needs to be provided, the environment must be managed, and feed and water must be supplied.
At least 24 hours before chick placement, the house and floor temperature are increased to a minimum of 34°C and 28°C, respectively. Proper ventilation and lighting are also essential. These conditions need to be monitored and adjusted after the placement so the chicks feel comfortable and start feed and water consumption. Checking chick behavior is crucial during the first hours after placement.
Upon the placement of the chicks, it is recommended to have pre-starter crumble feed available on top of brooder paper underneath the drinking line. To stimulate early feed and water consumption, gently place the chicks onto that paper. The target is to have 100 % of chicks with crop filled within 48 hours of chick placement.
Reduce the stocking density
 In general, high stocking density may restrict bird movement, interfere with airflow, and increase litter moisture and microbial growth, including pathogens, which potentially impairs broiler health, welfare, and performance.
In general, high stocking density may restrict bird movement, interfere with airflow, and increase litter moisture and microbial growth, including pathogens, which potentially impairs broiler health, welfare, and performance.
When reducing antibiotics, increase the space per bird by 0.05 ft2/46 cm2 per bird compared to your current conventional program. A lower stocking density helps keep litter moisture at a minimum, which reduces the shedding of cocci oocyst and pathogenic bacteria over the population.
Feed and water access must be granted to all animals at every moment. The number of chickens per feeder or drinker depends on the type of equipment used.
Consistent observation of the flock
To recognize emerging health issues, producers should critically observe the behavior of birds every day. On which points should they focus?
- First, when entering the house, birds’ behavior and response to the poultry worker should be observed with attention. Note the spread of birds throughout the house.
- Note birds’ drinking and eating behavior. Feed and water intake should be recorded daily, always at the same hour.
- The quality of the fresh fecal droppings should be judged. Any changes in the fecal droppings (loss of consistency) can help notice emerging disease and take measures against it.
Especially during and after feed change, attention to changes in the usual feces consistency is necessary.
Vaccination and judicious antibiotic use are crucial
Carefully consider vaccination programs for broilers. Unnecessary vaccinations impact the immune system, possibly resulting in reduced performance and, in some circumstances, make the birds more susceptible to other diseases. Hence, the vaccination program must be diligently attuned (Neetzon et al., 2017).
- The disease background of the parent farm as well as the broiler farm where the chicks will be placed are essential factors for the vaccination program
- If possible, vaccine strains that are the least immunosuppressive should be chosen
- If coccidiostats are not permitted, an effective vaccination against coccidiosis is required and must be done as early as possible
- All vaccinations must be given using a standard operating procedure that minimizes bird discomfort and optimizes the vaccine, and always administer vaccines following the advice from the manufacturer
After the vaccination, it is essential to monitor the effects of vaccination stress and take preventive measures to avoid any issues with broiler performance in terms of weight gain and mortality.
Use antibiotics with discernment
As we aim to reduce antibiotics, they should be limited to pure therapeutic use, only if other disease-prevention measures have not been successful, and mortality or disease symptoms make the treatment necessary. Before the treatment, the disease must be diagnosed by a qualified veterinarian. The diagnosis should be preferably followed up by isolation of the disease-causing bacteria, classification, and susceptibility testing before the antibiotics are applied.
Small-spectrum antibiotics that are less likely to cause antimicrobial resistance (AMR) should be preferred. Broad-spectrum antibiotics or antibiotics that are likely to cause AMR can only be used after susceptibility testing has demonstrated resistance to a first-choice antibiotic. The treatment effect must be evaluated by daily monitoring of disease symptoms, mortality, water, feed intake, and body weight gain.
Thinning – things to consider
If thinning (partial depopulation) is practiced, it should be done with the highest bio-security measures. Producers must ensure that the equipment used in the catching process is thoroughly cleaned before entering the house, and bird-catching personnel takes the same measures as farm personnel when entering the farm and the house. These policies will help to minimize the introduction of infectious agents.
Keep the feed withdrawal period for this process as short as possible to avoid flightiness, which can induce skin lesions (some regions catch birds in low light intensities to avoid flightiness). A short feed withdrawal period also prevents over-consumption of feed in a short amount of time, possibly disrupting feed passage in the gut and leading to bacterial imbalance and dysbacteriosis in the remaining birds. After thinning, feed and temperature must be adapted to the lower number of animals.
Provide your birds with high-quality water for drinking
 Water is the most important nutrient for broilers. It plays an essential role in digestion and metabolism, thermoregulation, and waste elimination.
Water is the most important nutrient for broilers. It plays an essential role in digestion and metabolism, thermoregulation, and waste elimination.
Several factors affect water quality: temperature, pH, bacteria, hardness, minerals, and total dissolved solids. These parameters should be analyzed at least twice per year. If necessary, corrective actions should be taken, e.g., a filtration to remove minerals, the addition of chlorine for disinfection, or the addition of organic acids to drop the pH.
Before each cycle, the water must be tested for total aerobic + enterobacteria, compared to reference values: Total plate count (TPC) should be < 1000 CFU/ml, and E.coli, Enterobacteriaceae, yeast, and molds at undetectable levels. The section about cleaning and disinfection of the waterline provides insights and practical advice about water sanitation and microbiological analysis.
Nutrition & feeding – a pillar for antibiotic reduction
Nutrition and feeding in ABR broiler production are not only about providing nutrients for growth but also about the effects of the feed on gut health. Gut health is essential for animals’ overall health, welfare, and productivity, even more so in antibiotic reduction scenarios.
Feed should be of the highest quality – in all respects
High feed quality is necessary to provide the animal with the required nutrients and achieve their optimal utilization. Also important is the absence, limitation, or management of harmful substances and pathogens. High quality, therefore, includes:
- Form and composition of the final feed
- Nutritional value of the raw materials
- Management of harmful substances.
From reception and storage of the raw materials to the dispatch of the finished feed, the feed mill management emphasizes their quality assurance system, which is decisive in this connection.
First measure: quality assurance at the feed mill level
The feed mills producing for operations with no or reduced use of antibiotics must have a quality assurance (QA) and/or a good manufacturing program (GMP) in place that guarantees the production of consistently good quality feeds.
Proper raw material management and processing of feeds are necessary to achieve the lowest possible microbial-pathogen load, including:
- An effective rodent and wild birds control
- Disinfection of all the vehicles entering the feed mill
- Proper storage and utilization of raw materials (e.g., first in-first out use, silo management)
- Periodic thorough cleaning of milling equipment, premises and storage areas, and the monitoring of these activities
- Standard operating procedure and quality assurance systems that guarantee feed safety and quality
Check the quality of the raw materials and the final feed
Digestion, absorption, and gut health depend on the quality of the feed ingredients. To provide the best preconditions for healthy growth, producers should avoid raw materials of a reduced and/or inconsistent quality. For this purpose, each raw material batch should be analyzed for its specific quality parameters. Quality parameters to consider are:
- Physical ones, such as color, odor, particle size, and general appearance
- Chemical ones, such as nutritional composition and specific parameters. For example, grains should be analyzed for mycotoxins and antinutritional factors; fats and oils need to be analyzed for free fatty acids (FFA), unsaturated/saturated (US) ratio, iodine value (IV), but also the peroxide value (PV) as oxidized fats have a lower energy value, and their intake is related to enteric diseases
- Biological ones, including yeasts, molds, and enterobacteria
Also, the finished feed should be monitored by analyzing every batch concerning composition compared to values in the feed formulation, as well as physical, chemical, and microbiological quality parameters.
Clean storage on the farm prevents feed spoilage
As in the feed mill, keeping the farm facilities clean is of the highest importance. Warehouses, silos, bins, feeders, etc., should be emptied, cleaned, and disinfected after each flock; this avoids the formation of feed aggregates that can lead to mold growth and mycotoxin contamination; also, insects, bacteria, and parasites can remain in those residues.

Adapt feed formulation and feeding to the feeding phase
The value of phase feeding
Having the correct number of dietary phases to meet animal demands and avoid excess nutrients provides better intestinal health and thus aids production animals in ABR scenarios. The feeding phases should be designed to prevent abrupt changes in nutrition and raw material inclusions, possibly leading to dysbacteriosis.
Feeding for gut health
When feeding broilers in antibiotic reduction scenarios, extra care should be taken when formulating diets. The challenge is to achieve the same performance as conventional management at an optimum cost.
- Don’t waste nutrients: Improve feed digestibility, and at the same time, reduce the dangers of antinutritional factors coming from different ingredients by using suitable exogenous enzymes.
- Keep an eye on fiber: Moderate levels of insoluble fibers with adequate structure and composition can be included to promote gizzard development and function. This measure leads to a better modulation of gut motility and feeds passage into the intestine. Additionally, it promotes gut health, resulting in higher nutrient digestibility.
- Be careful with protein: Excess of undigested protein in the hindgut may lead to the proliferation of Clostridium perfringens; then, subclinical challenges of necrotic enteritis may occur. Moreover, the excess of nitrogen may increase feces moisture content, leading to wet litter. The optimization of the diets based on digestible amino-acid profiles and the use of synthetic amino acids decrease or eliminate the minimum requirements of crude protein, avoiding its excess.
Which feed form?
The feed form depends on the age or feeding phase: starter feeds can be offered as coarse mash, but preferably as crumble or mini-pellets (< 2 mm diameter) and grower and finisher diets as 3 – 4 mm pellets.
When using pelleted diets, quality is also the most crucial criterion. Poor pellet quality and thus the excess of fine particles increase feed passage rate, resulting in poor gizzard development and compromised gut health.
A high-quality pelleted feed can withstand – without much breakage – the handling that occurs after processing, such as transportation, storage, and farm management. Pellet quality can be measured by the Pellet Durability Index (PDI) obtained by simulating the impact and shear forces in a known quantity of feed for a determined amount of time. After this time, the sample is sieved, and the fines are separated, weighed, and compared with the initial sample
The PDI should be measured in the feed mill and compared to a standard. Later, it is also recommended to measure the PDI on the farm, and the producer should take corrective actions if the pellets cannot maintain their quality.
Additionally, it should be known that coarse ground grains stimulate gizzard development and function. So, about 30 % of the feed should consist of particles between 1-1.5mm (post pelleting) in all feeding phases.
Broilers’ selection criteria for feed are form, color, size,
|

Broilers’ selection criteria for feed are form, color, size, and consistency. They prefer feed that is easy to pick, such as crumbles or pellets.
Feed additives can support antibiotic reduction
The feed additive industry provides broiler farms and integrations with various solutions to make production more manageable and efficient.
A healthy start is half the battle
Let’s start with the chicks. The early introduction of beneficial bacteria into the intestinal tract has proven helpful for gut health optimization. This colonization can be achieved with the administration of suitable probiotics preparation at the hatchery. Multi-strain probiotic preparations effectively initiate healthy microbiome development for optimum gut health. For these challenges, support is offered through EW Nutrition’s VENTAR D and ACTIVO LIQUID, phytomolecule-based products for the feed and the waterline, respectively.
Maintain gut health
Gut health is one of the essential preconditions for efficient growth. Only a healthy gut guarantees efficient digestion and absorption of nutrients. Several approaches are recommended to maintain gut health:
- Promotion of beneficial and reduction of pathogenic gut flora: here, solutions can come in the shape of products based on phytomolecules that can be applied with the feed (VENTAR D) or the water (ACTIVO LIQUID)
- Management of bacterial toxins and mycotoxins: for this topic, products mitigating the toxins’ negative impact on the birds (Product range of MASTERSORB and SOLIS) are offered
Protect your feed
When feed is stored, there is always the risk of bacteria, mold, or yeast overgrowth. Oxidation of feed ingredients, such as fats and oils, reduces their nutritional value. These issues can be prevented by applying:
- Acidifiers that have antimicrobial effects due to their pH-decreasing effect, which, later on, improves the feed digestibility and stabilizes the GIT flora (ACIDOMIX, FORMYCINE, and PRO-STABIL)
- Antioxidants preserving ingredients susceptible to oxidation, such as fats and oils (AGRADO, SANTOQUIN, and STABILON)
Improve pellet quality
Moisture retention during the conditioning process influences pellet quality: higher moisture retention entails a higher starch gelatinization resulting in higher digestibility, pellet binding, fewer fines, and a higher PDI. Surfactants (for example, SURF•ACE) are compounds that can reduce the surface tension between the water and the feed, improving moisture absorption during the conditioning process.
Besides that, moist steam in the pelleting process penetrates better and has a higher antimicrobial effect leading to lower production of bacterial and mycotoxins. The possible reduction of the pelleting temperature protects the nutrients.
ABR in broiler production is practicable – by observing some rules
As shown above, antibiotic-reduced broiler production needs many aspects to be considered and a lot of measures to be taken. All of these measures seek to keep animals healthy and avoid antibiotic use. Maintaining gut health is crucial, as only a healthy gut performs well, achieves the optimal utilization of nutrients, and increases growth performance.
Maintaining a successful production unit with no or reduced antibiotic use requires a holistic approach in which best practices must be assured at all levels of the production chain. The feed additive industry provides a broad range of solutions to support animal production through this challenging task. The objective could not be more critical: lowering antibiotic resistance to assure the future of animal and human health.
References:
Davies, Robert, and Andrew Wales. “Antimicrobial Resistance on Farms: A Review Including Biosecurity and the Potential Role of Disinfectants in Resistance Selection.” Comprehensive Reviews in Food Science and Food Safety 18, no. 3 (2019): 753–74. doi.org/10.1111/1541-4337.12438
Dewulf, Jeroen, and Van Filip Immerseel. “General Principles of Biosecurity in Animal Production and Veterinary Medicine.” Essay. In Biosecurity in Animal Production and Veterinary Medicine: From Principles to Practice. Wallingford, Oxfordshire, UK: CABI, 2019. doi.org/10.1079/9781789245684.0063.
Luyckx, K.Y., S. Van Weyenberg, J. Dewulf, L. Herman, J. Zoons, E. Vervaet, M. Heyndrickx, and K. De Reu. “On-Farm Comparisons of Different Cleaning Protocols in Broiler Houses.” Poultry Science 94, no. 8 (2015): 1986–93. doi.org/10.3382/ps/pev143.
Kreis, Anna. “Broiler Feed Form, Particle Size Assists Performance.” Feed Strategy, September 20, 2019. https://www.feedstrategy.com/poultry-nutrition/broiler-feed-form-particle-size-assists-performance/.
Malone, B. “Litter Amendments: Their Role and Use.” University of Delaware – Agriculture & Natural Ressources – Fact Sheets and Publications. University of Delaware, November 2005. https://www.udel.edu/academics/colleges/canr/cooperative-extension/fact-sheets/litter-amendements/
Neetzon, A. M., Pearson, D., Dorko, N., Bailey, R., Shkarlat, P., Kretschmar-McCluskey, V., Van Lierde, E., Cerrate, S., Swalander, M., Vickery, R., Bruzual, J., Evans, B., Munsch, G., & Janssen, M. (2017, October). Aviagen Brief. Aviagen – Information Library. https://en.aviagen.com/assets/Tech_Center/Broiler_Breeder_Tech_Articles/English/AviagenBrief-ABF-Broiler-EN-17.pdf.
UC Davis Veterinary Medicine. “‘All out All in’ Poultry Management Approach to Disease Control. A Guide for Poultry Owners.” Poultry-UC ANR, March 2019. https://ucanr.edu/sites/poultry/files/301023.pdf