INFOGRAPHIC: Healthy piglets after weaning



By Technical Team, EW Nutrition
A storm has been brewing.
Even before the invasion of Ukraine in late February, global growth was expected to trend significantly downward, from 5.5-5.9% in 2021 to 4.1-4.4% in 2022 and 3.2% in 2023. The causes are similar across industries:
In early 2022, this “perfect storm” quickly stifled the moderate optimism of Q4 2021. Of course, the worst was yet to come.
With the ongoing crisis in Eastern Europe, economic perspectives are tilting down to a new level of uncertainty. The new variables now thrown into the mix are crude oil and natural gas prices, as well as added concerns over other raw materials coming out of Russia and Ukraine.
Source: tradingeconomics.com, March 2022
Russia accounts for 25% of the global natural gas market and 11% of the crude oil market. It is also the largest wheat exporter (China and India are still the largest producers, but Russia exports appreciably more). Together with Ukraine, also a powerhouse of agricultural exports, the two now enemies account for 29% of international annual wheat sales.
Source: ING, March 2022
Wheat prices were already nearly double the five-year average shortly before the invasion; after February 24, they rose by another 30%. Today we are at a staggering 53% increase in wheat prices in just the last few months. We are at a 14-year peak. And the countries that import the most from Russia and Ukraine (such as Egypt or Indonesia) will bear the brunt of this crisis.
Together, Russia and Ukraine’s exports account for 12% of the world’s traded calories. The two countries account for almost 30 percent of global wheat exports, almost 20 percent of corn exports, and more than 80 percent of the world supply of sunflower oil. However, the compounded effect of embargo and devastation in the two countries will surely exert tremendous influence on the global economic outlook for years to come.
Agriculture was already hurting before February 24th. Poor harvests caused by extreme weather conditions, continued losses along the production chain, supply chain issues, and abnormal pandemic buying patterns combined to sink global wheat stocks one third lower than the five-year average. Reserves, in other words, are low – and will be significantly lower.
We need to be realistic about the coming months and years. Corn (where Ukraine accounts for 13% of global exports) and wheat will be severely hit by the war and its aftermath. This will compound all the pre-existing factors (transportation costs, supply chain slowdown, continuing weather disruptions, energy costs), none of which will trend down. Fertilizer prices have also gone up exponentially, and Russia – the largest exporter – has banned fertilizer exports at the beginning of March. The effects will be ultimately reflected in the cost of raw materials.
Ukraine and Russia have all but banned grains exports – either for security reasons or to protect internal needs. On top of this, the last harvests collected in Ukraine are now sitting in bins where ventilation and temperature controls have been affected by power cuts.
Source: World Bank, March 2022
At the end of February, World Bank data already showed upward movement for nearly all categories; whatever was not trending up at that time is catching up fast. The last time things looked like this, experts warn, was in 2008-2009 – and social unrest followed around the world, to serious global consequences.
However, the perspective is not catastrophic and there is room to conserve profitability. The essential is to intervene with fast, targeted action that favors smart optimization, localization, and long-term planning.
Most feed producers will be caught in the middle of all rising costs, from raw materials to transport and energy. Where, then, can they look for shelter when the storm hits?
One of the first things feed producers will focus on will be cutting down feed costs. At this point, it is essential that this basic optimization does not impact animal health and performance. Here is what should be kept in mind.
Whatever raw materials you choose to use, minimizing losses and maintaining quality should be the first step. Losses caused by storage are often the easiest to mitigate.
Quick intervention #1: Use mold inhibitors and mitigate the impact of mycotoxins
Freight costs will continue to cause pressure on transported raw materials, driving producers to local/regional options. When you replace one feed ingredient with a cheaper one, the first effects will be on the active principle and on the digestibility of the feed. Often something you are taking out of the diet cannot be replaced 1:1.
Quick intervention #2: Maximize the use of enzymes to ensure high feed digestibility; for poultry, pigments can replace corn-derived coloration (to control color variability)
Adjusting the feed composition doesn’t only have effects on paper.
Even if you choose the best replacements, adjust the balance, compensate for loss of digestibility and optimize everything in every possible way, one thing remains:
The animal receives a new diet.
New diets are textbook stressors. But sometimes the nutritionist or the producer is so stressed that it is easy to overlook the stress placed inside the animal. Since animal efficiency is key for productivity, it is essential that the effects of diet stress are mitigated for the animal.
Quick intervention #3: Precautionary use of gut-health mitigating additives; also consider palatable feed materials and taste enhancers
To optimize costs on the production floor, there are three essential areas where feed producers can act:
To answer these challenges, there are solutions that can operate individually. More importantly in such times, there are products that can impact all three areas without negatively influencing the quality of output. One such solution, for instance, can decrease energy costs, increase throughput and pellet quality, and reduce fines.
Quick intervention #4: Choose a solution that satisfies 3/3 of your issues
Climate change will continue to wreak havoc on the predictability of harvests. Freight costs are projected to keep rising. And the costs of war and (hopefully) reconstruction will take a toll on the cost of living and cost of doing business around the world, for years to come.
In the storm that has already started, it is unwise to take shelter for a while and hope for good weather soon. Cutting down on ingredients here and additives there won’t keep profitability high in the long run. Feed producers must look at all aspects – from feed storage and composition to process improvement – and consider holistic measures that protect animals and profitability at the same time.

Poultry producers worldwide use natural carotenoids in feed formulations for laying hens and pigmented broilers. With European Union regulation restricting the use of apoester to 5 ppm in animal feed, it is more relevant than ever for poultry producers that safe, natural alternatives exist. Regulatory limits for natural xanthophylls, in contrast, are set at up to 80 ppm in complete feed.
At EW Nutrition, natural xanthophyll production is a specialized and standardized process that includes quality assurance at all stages, from planting to harvesting, extraction, and saponification. The outcome is uniform and very stable products that deliver consistent, reliable results.
How to choose and handle pigmentation products for maximum performance?
EW Nutrition’s Colortek Yellow B pigment for poultry contains ≥ 100 g/kg of natural yellow xanthophylls extracted from the marigold flower (Tagetes erecta spp.). It achieves consistent, uniform, and high-quality coloration for egg yolk and broiler skin, as attested by independent certifications FAMI QS, ISO 14000, and ISO 9001.
A trial was designed to compare the stability of natural Colortek Yellow B and a synthetic apoester product (Carophyll Yellow, DSM [Batch L 1954]) in a premix under challenge conditions (high level of choline chloride). As shown in Figure 1, Colortek Yellow B outperformed the apoester, offering superior stability.
Figure 1. Stability in vitamin mineral premix (12.5% choline chloride, closed bag, 30°C, 75% RH)
These results underscore that Colortek Yellow B offers the stability poultry producers require for a successful pigmentation program. As poultry producers adopt natural carotenoid alternatives, they can be assured that specialized and standardized production processes and strict quality controls guarantee these products’ reliable performance.

By Dr. Inge Heinzl, Editor, EW Nutrition
Salmonella infection in poultry is a problem for the producer because of the performance losses of his flock. At the same time, products of salmonella-contaminated animals pose a severe risk to human health. In the USA, Salmonellosis in poultry is estimated to cost $ 11.6 billion each year (Wernicki et al., 2017) and more than € 3 billion in the EU (Ehuwa, 2021). As the use of antibiotics needs to be reduced to keep them effective, Salmonella control in poultry requires new solutions. This article shows how organic acids and phytomolecules can help to fight this problematic disease.
Salmonellosis is a zoonosis, meaning that it can be easily transferred from animals to humans. The transfer can occur via different routes:
Frozen or raw chicken products, as well as the eggs of backyard hens, are the most frequent causes of animal-mediated Salmonella infections in humans. The following graphic shows a clear relationship between the occurrence of Salmonella in layer flocks and the event of disease in humans:
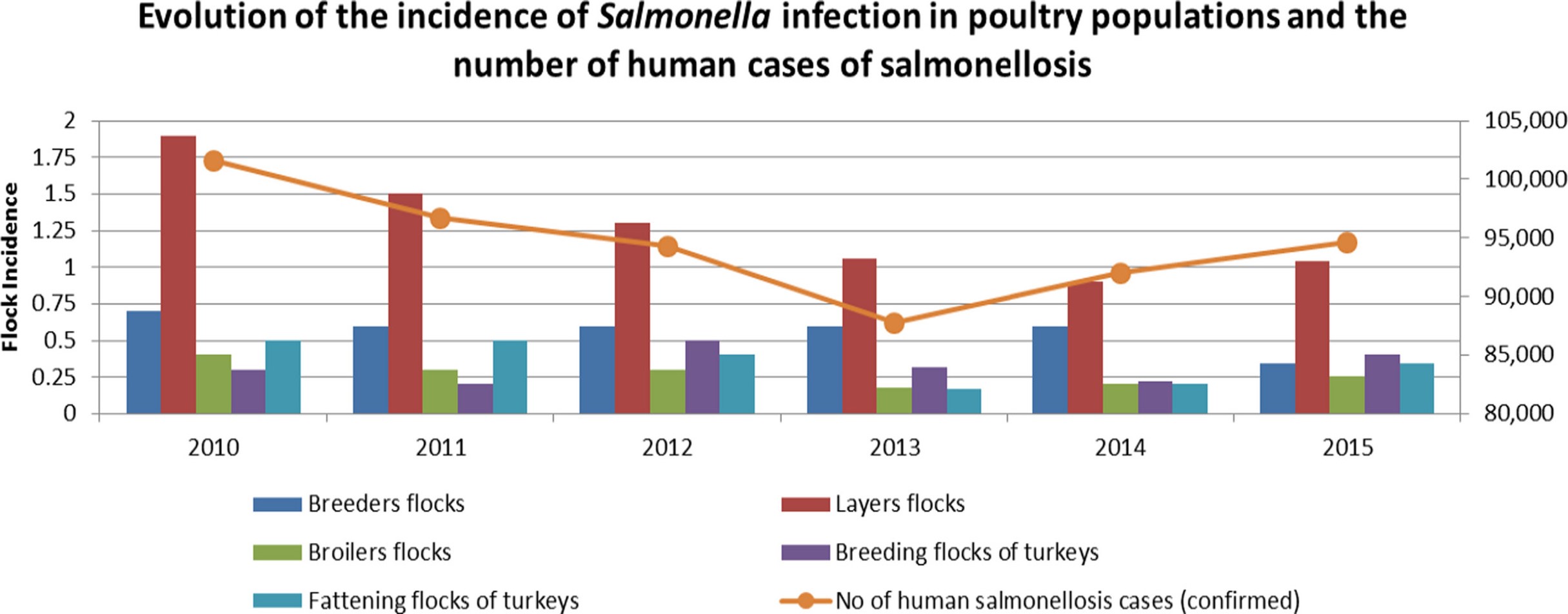
Within the poultry flock, there are two ways of spreading: the fecal-oral way (horizontal infection) or the infection of the progeny in the egg (vertical infection). The effects of the disease depend on the age of the birds: the younger the animals, the more severe the impact.
If the brood eggs already carry salmonellae, the hatchability dwindles. During their first month of life, infected chicks show ruffled downs and higher temperatures. Diarrhea leads to fluid losses and frequently to the chicks’ death.
Adult animals usually do not die from Salmonellosis; often, the infection remains unnoticed. During a substantial acute salmonella outbreak, the animals show weakness and diarrhea. They lose weight, resulting in decreased egg production in layers and worse growth performance in broilers. The birds need more water to compensate for the fluid losses, and their crowns and jowls appear pale.
Salmonellae have developed a clever way to protect themselves. After they arrive in the gut, they attach to the epithelial cells and form small molecular “syringes” to inject divers substances into the gut cells (Type-3-injection system). These signaling substances make the gut cells bulge their membranes and enclose the bacterium. Finally, the manipulated gut cell absorbs the Salmonella, the host “allows” the bacterium to enter, and it can proliferate in the gut cells (Fischer, 2018).
When an antibiotic is attacking the bacterium, Salmonellae stop their cell division. Since many antibiotics are only effective against bacteria during cell division and growth, Salmonellae survive the attack by staying as dormant variants or persisters until the treatment stops (Fischer, 2018).
The genus of Salmonella consists of more than 2600 serovars (Ranieri et al., 2015), of which less than 100 are relevant for humans (CDC, 2020). More than 1500 serovars belong to the Salmonella enterica subspecies that colonize the intestinal tract of warm-blooded animals. These serovars are responsible for 99 % of salmonella infections (Mendes Maciel et al., 2017). The main serovars relevant for poultry are S. Gallinarum and S. Pullorum, but also S. Enteritidis, Typhimurium, and in recent years, S. Kentucky, S. Heidelberg, S. Livingstone, and S. Mbandaka (Guillén et al., 2020).
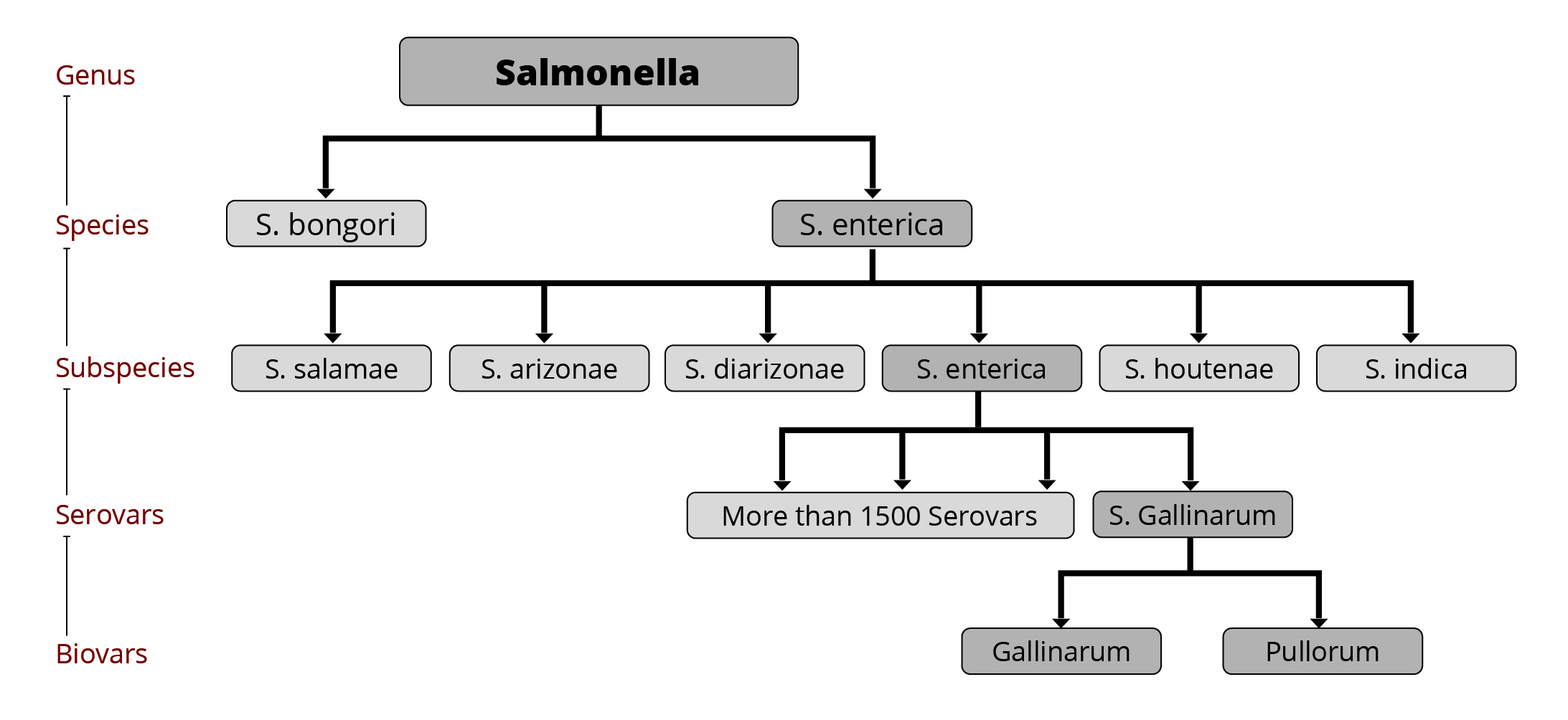
Several Salmonella serovars are critical for animals and humans. Since more than 91,000 salmonellosis cases are reported for Europe and more than 1.35 million for the USA every year (EFSA, 2022; FDA, 2020), their spread must be prevented by all means. Governments have enacted some laws to curtail this disease. The EU, for example, implemented extended control programs for zoonotic diseases, with Salmonella set as a priority. These programs include the provision of scientific advice, targets for reducing Salmonella in poultry flocks, and restrictions on the trade of products from infected flocks.
For farmers and vets, this means the obligation to notify the occurrence of the disease to the authorities. Depending on the country, it also entails compulsory vaccination and the documentation of hygienic measures. In the EU, due to the risk of developing resistances, the EFSA recommends limiting the use of antimicrobials to individual cases, e.g., to prevent inordinate suffering of animals.
The best strategy for salmonella control is prevention based on three key points (Visscher, 2014):
For this purpose, the following steps are requested/recommended:
The use of well-absorptive material such as wood shavings, straw pellets, or straw granulates and regular removal of the used litter is recommended. The animals must be controlled for diarrhea to avoid wet droppings. The water supply must be adequate; an excessive water supply wets the litter.
To keep the poultry house clean, broken eggs and dead animals (potential sources of infection) must be removed. In general, the houses should be cleaned and disinfected before every restocking.
Clean feed and water are essential; therefore, feed should not be stored outside but be kept dry and protected from pests and rodents. The feeding of the animals should take place inside to avoid contamination by wild birds. Concerning the water for drinking, the flow rate must be high enough to provide the birds with sufficient water but not too high that the floor gets wet. The troughs must be clean from droppings.
To limit the spread of Salmonella, only a restricted number of persons can have access to the flocks. They must wear clothes, and instruments should be exclusively used for the respective poultry house.
Salmonellae are a genus in the family of Enterobacteriaceae. They are gram-negative, rod-shaped (size: approx. 2 µm), glucose-fermenting facultative anaerobes that are motile due to peritrichous flagella. Since Salmonellae do not form spores, they can be easily destroyed by heating them to 60°C for 15-20 min (Forsythe, 2001), especially in food/feed with higher water content.
For the storage of food, Bell and Kyriakis (2002) found that most serovars of Salmonella will not grow at temperatures lower than 7°C and a pH lower than 4.5. Wessels et al. (2021) showed optimal growth conditions for Salmonella: temperatures between 5 and 46°C (optimum 38°C), a water activity of 0.94-0.99, and a pH of 3.8-9.5.
A high fat content in the feed or food increases the likelihood of infection with Salmonella because the fat protects the bacteria during the passage through the stomach. Doses of 10 to 100 Salmonella cells can already pose a severe risk (University of Georgia, 2015).
To reduce the incidence of Salmonella while simultaneously lowering the use of antibiotics in animal production, there are different possibilities. On the one hand, veterinary medicine offers vaccines. On the other hand, the feed industry provides additives that strengthen the immune system, improve gut health, or support the animals in another manner. Other than pro- and prebiotics, the main active ingredient categories for such additives are organic acids and phytomolecules.
Already in ancient Egypt, the method of fermentation and the generated acids have been used for the conservation of food (Ohmomo et al., 2002). Nowadays, it is a standard tool to protect feed (silage) and food from spoilage. Also for animals, organic acids added to the feed or the water have proven helpful against pathogens. These modes of action can be combined against Salmonella: reducing the pathogen load in the feed to limit the intake of bacteria and fighting against these pathogens in the animal.
In general, the antimicrobial activity of organic acids in feed is based on lowering the pH (Pearlin et al., 2019). pH-sensitive bacteria such as Salmonella minimize their proliferation at a pH <5. Additionally, the organic acids attack bacteria directly. The acid’s undissociated and more lipophilic form penetrates the bacterial cell membrane. At the neutral pH within the cell, the acid dissociates, releases protons, and lowers the pH, leading to the impediment of metabolic processes in the cell. The cell spends a lot of energy trying to get the pH back to neutral (Mroz et al., 2006). Additionally, the anions become toxic for the cell metabolites and disrupt the membrane (Russel, 1992).
According to Hernández and co-workers (2006) and Thompson and Hinton (1997), the addition of organic acids to the feed does not change the pH in the various digestive tract segments. Still, literature shows a clear reduction of Salmonella in the gut or litter when using propionic or/and formic acid (McHan and Emmett, 1992; Hinton and Linton, 1988; Humphrey & Lanning, 1988). A likely mode of action is described by Van Immerseel et al. (2004). He asserts that SCFAs such as propionic and formic acid as well as MCFAs can inhibit Salmonella’s penetration of the intestinal epithelium and, therefore, can control these invasive phenotypes of Salmonella (S. Typhimurium and S. Enteritidis).
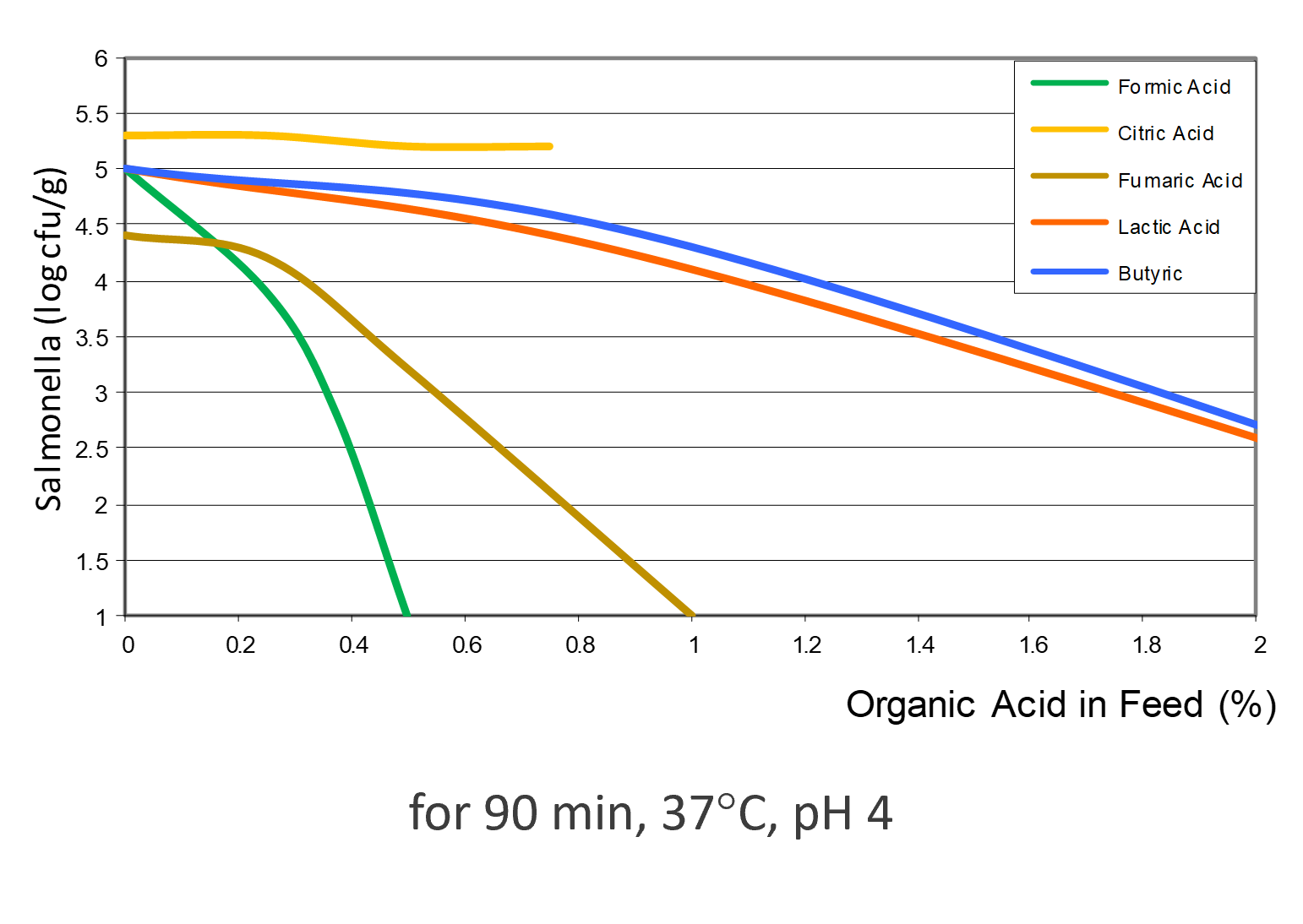
Depending on the acid, the efficacy against Salmonella varies (see figure 3). Formic acid shows the highest effect, followed by fumaric acid. Then, lactic, butyric, and citric acid follow, showing lower efficacy.
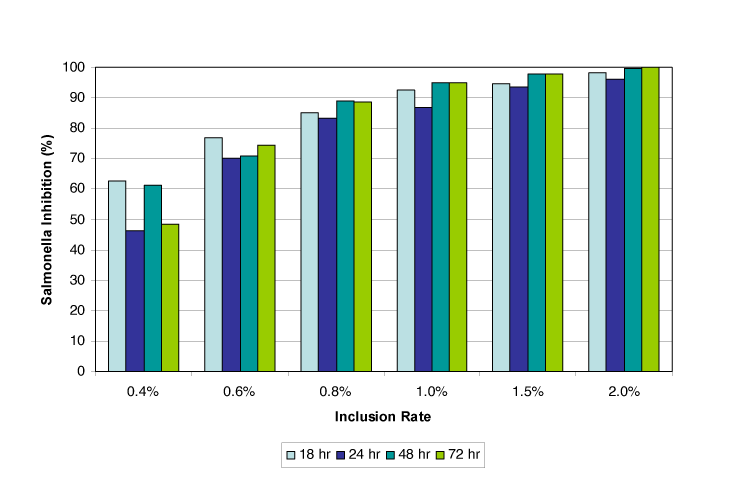
An in-vitro trial was conducted at a commercial research facility in the US to test the efficacy of Acidomix AFL, a liquid mixture of propionic and formic acid, against Salmonella. The bacterial strain used in these studies was nalidixic acid-resistant Salmonella typhimurium. The bacteria were maintained in broth cultures of tryptic soy broth.
They were added to 5 g of dry feed in a 50 ml tube to a final concentration of 40,000 CFU/g. Next, Acidomix AFL was added to the desired inclusion rate, and the samples were incubated at room temperature. After 18 to 72 hours of incubation, viable bacteria were counted using the plate count method.
Results: As shown in figure 4, the trial found that at an inclusion rate of 2.0 %, Salmonella inhibition was nearly 100 %. Already at a 0.4 % inclusion rate, Salmonella could be reduced by 45-60 %, showing a clear dose dependency.
Plants produce phytogenic substances to protect themselves from molds, yeasts, and bacteria, among others. After several purification steps, these phytomolecules can be used to fight Salmonella in poultry. They work through different modes of action, from attacking the cell wall (terpenoids and phenols) to influencing the genetic material of the pathogenic cells or changing the whole morphology of the cell.
Due to the different modes of action, it was long thought that there would be no resistance development. Still, Khan et al. (2009) found some microorganisms such as multidrug-resistant E. coli, Klebsiella pneumoniae, S. aureus, Enterococcus faecalis, Pseudomonas aeruginosa, and Salmonella typhimurium can show a certain – perhaps natural – resistance to some components of herbal medicines.
Gram-negative bacteria such as Salmonella are usually less attackable by phytomolecules because the cell wall only allows small hydrophilic solutes to pass; however, phytomolecules are hydrophobic. However, mixing the phytomolecules with an emulsifier facilitates the invasion into the cell. Their efficacy depends on their chemical composition. It is also decisive if single substances or blends (possible positive or negative synergies) are used.
The best-clarified mode of action is the one of thymol and carvacrol, the major components of the oils of thyme and oregano. They can get into the bacterial membrane and disrupt its integrity. The permeability of the cell membrane for ions and other small molecules such as ATP increases, decreasing the electrochemical gradient above the cell membrane and the loss of energy equivalents.
Two different phytogenic compositions were tested for their efficacy against Salmonella.
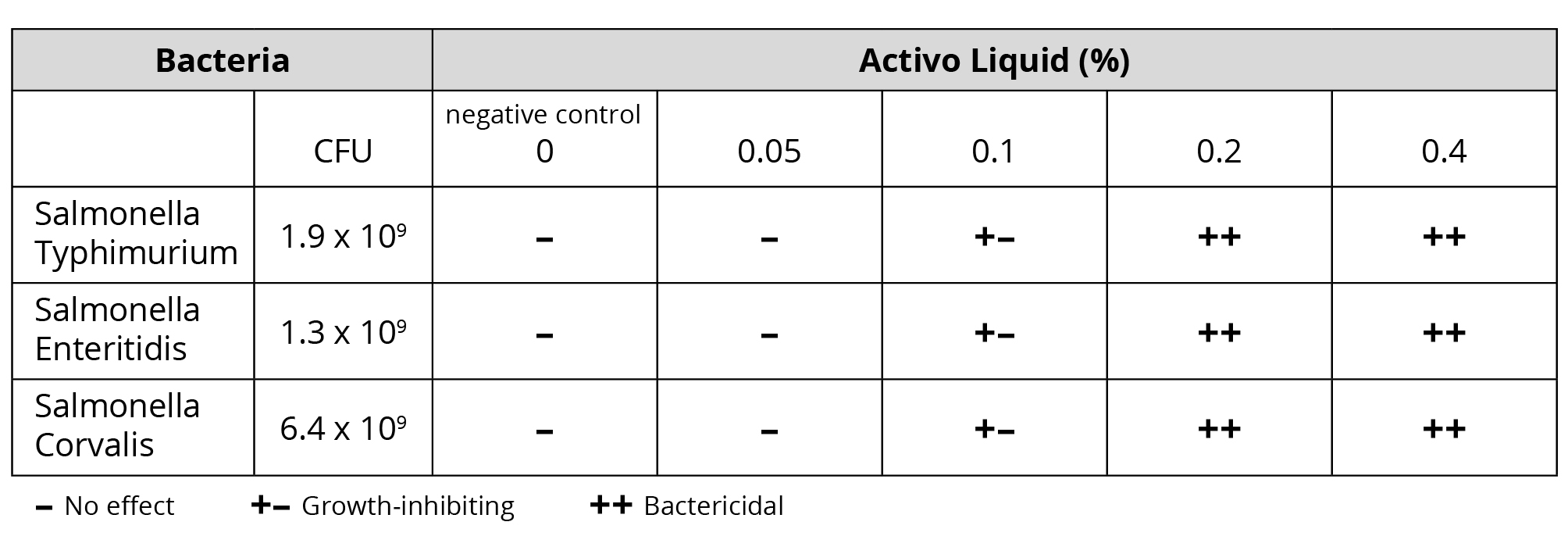
To evaluate its potential as a tool for antibiotic reduction, a trial was conducted to test the antimicrobial properties of Activo Liquid, a mixture of selected phytomolecules and an organic acid designed for application in water. The laboratory test was carried out at the Veterinary Diagnosis Department of Kasetsart University in Thailand. Standardized suspensions [1×104 CFU/ml] of three poultry-relevant Salmonella strains were incubated in LB medium, either without or with Activo Liquid. The tests were run at concentrations of 0.05%; 0.1%; 0.2% and 0.4%. After incubation at 37°C for 6-7 hours, serial dilutions of the cell suspensions were transferred onto LB agar plates and incubated for 18-22h at 37°C. Subsequently, colonies (CFU/ml) were determined.
Results: Activo Liquid was found to be growth-inhibiting to all Salmonella strains from a concentration of 0.1% onwards. At 0.2%, Activo Liquid already exhibited bactericidal efficacy against all tested Salmonella isolates, which was confirmed at a concentration of 0.4%.
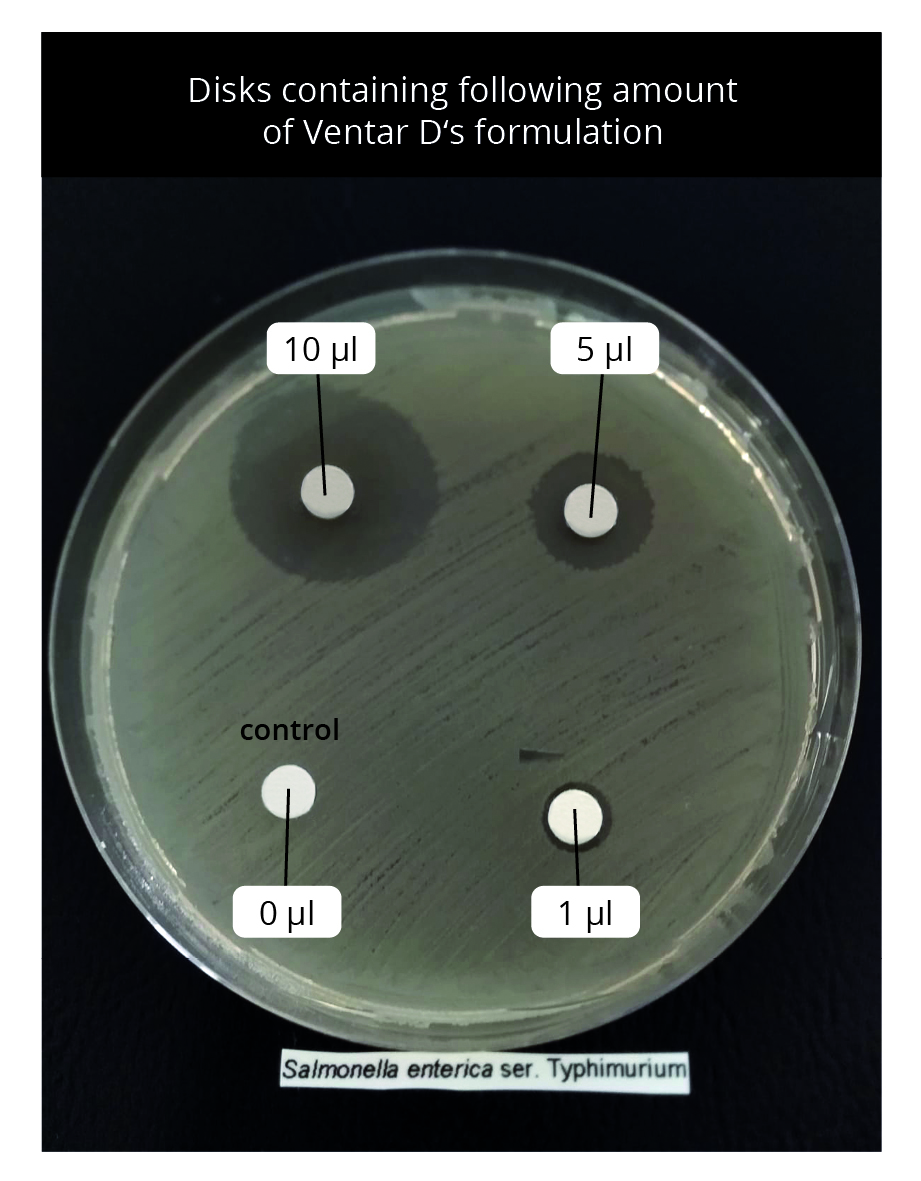
On Mueller Hinton agar plates where Salmonella enterica were spread uniformly, small disks containing 0 (control, only methanol), 1, 5, and 10 µl of Ventar D were placed and incubated at 37 °C for 16 hours. The presence of clearing zones indicates antimicrobial activity.
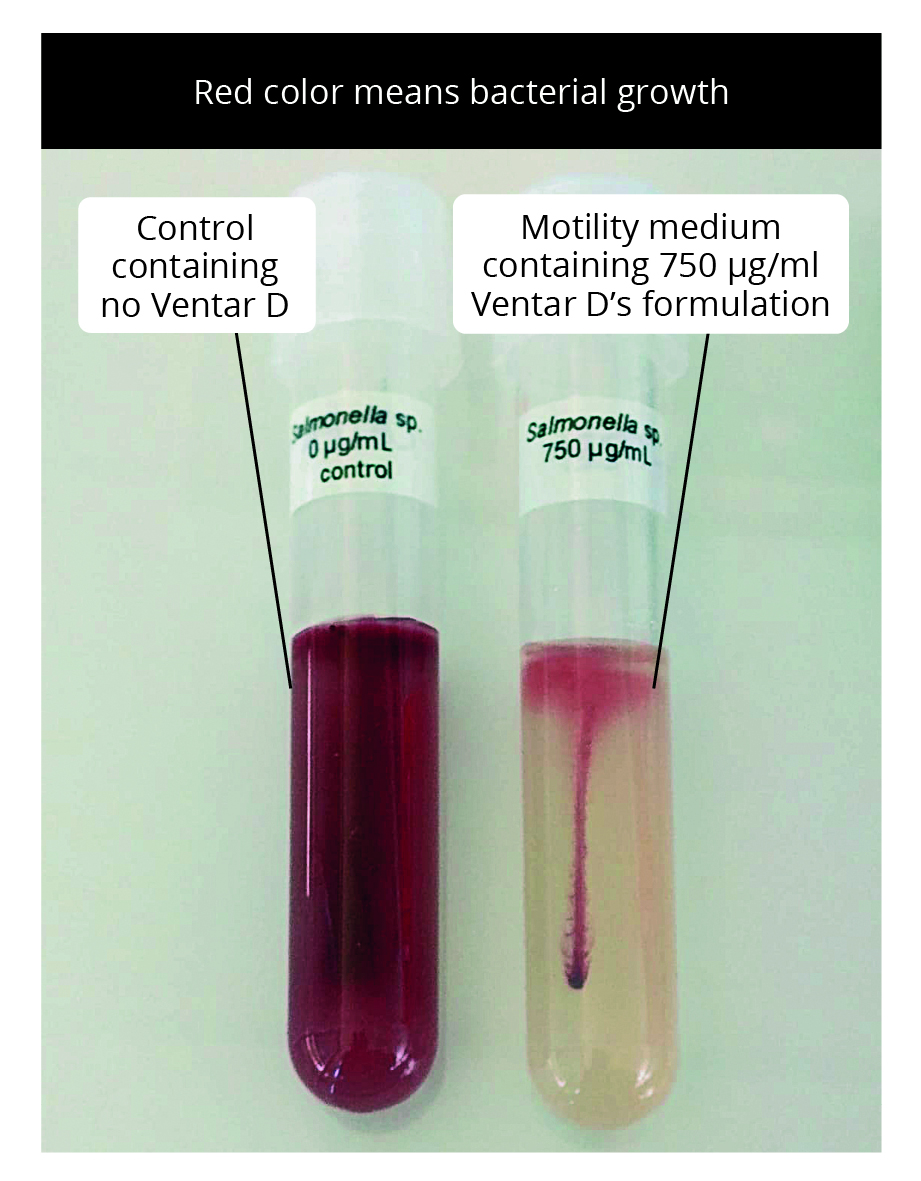
Additionally, a motility test was performed in tubes with a motility test medium containing 0 (control) and 750 µL Ventar D. For this purpose, one colony of Salmonella enterica grown on the agar was stuck in the middle of the medium and incubated at 37 °C for 12-16 hours. Growth can be visualized through the formation of red color.
Result: Ventar D inhibited S. enterica in a dose-dependent manner. Clearing zones were visible within the lowest tested concentration. At its inhibitory concentration, Ventar D suppressed S. enterica motility (figures 5 and 6).
The zoonosis Salmonella generates high costs in the poultry industry. As Salmonellosis can be transferred to humans, it must be kept under control by all means. Antibiotics are one tool to fight Salmonella, but they have their “side effects”: they are no longer well respected by the consumer, and, even more critically, they create resistance. To help keep antibiotics effective, poultry producers seek to use effective but not resistance-creating natural solutions against Salmonella.
As shown with the reviewed trials, organic acids and phytomolecules are highly active against diverse Salmonella serovars. Accordingly, feed additives based on these active ingredients offer effective tools for controlling Salmonella in poultry while also contributing to the overarching aim of reducing antibiotic use in poultry production.
Bell, Chris, and Alec Kyriakides. Salmonella: A Practical Approach to the Organism and Its Control in Foods. Oxford: Blackwell Science, 2002.
Castro-Vargas, Rafael Enrique, María Paula Herrera-Sánchez, Roy Rodríguez-Hernández, and Iang Schroniltgen Rondón-Barragán. “Antibiotic Resistance in Salmonella Spp. Isolated from Poultry: A Global Overview.” October-2020 13, no. 10 (October 3, 2020): 2070–84. https://doi.org/10.14202/vetworld.2020.2070-2084.
CDC. “Serotypes and the Importance of Serotyping Salmonella.” Centers for Disease Control and Prevention, February 21, 2020. https://www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotyping-importance.html.
EFSA. “Salmonella.” European Food Safety Authority. Accessed February 1, 2022. https://www.efsa.europa.eu/en/topics/topic/salmonella.
Ehuwa, Olugbenga, Amit K. Jaiswal, and Swarna Jaiswal. “Salmonella, Food Safety and Food Handling Practices.” Foods 10, no. 5 (2021): 907. https://doi.org/10.3390/foods10050907.
FDA. “Get the Facts about Salmonella.” U.S. Food and Drug Administration, July 28, 2020. https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-about-salmonella.
Fischer, Andreas. “Clever Infiziert – Die Tricks Der Bakterien.” HZI – Helmholtz Zentrum für Infektionsforschung, August 19, 2021. https://www.helmholtz-hzi.de/de/aktuelles/thema/clever-infiziert-die-tricks-der-bakterien/.
Forsythe, Steve J. The Microbiology of Safe Food. Hoboken, NJ: Wiley-Blackwell, 2020.
Gheisari, A.A., M. Heidari, R.K. Kermanshahi, M. Togani, and S. Saraeian. “Effect of Dietary Supplementation of Protected Organic …” WPSA, 2007. https://www.cabi.org/Uploads/animal-science/worlds-poultry-science-association/WPSA-france-2007/74.pdf.
Guillén, Silvia, María Marcén, Ignacio Álvarez, Pilar Mañas, and Guillermo Cebrián. “Stress Resistance of Emerging Poultry-Associated Salmonella Serovars.” International Journal of Food Microbiology 335 (2020): 108884. https://doi.org/10.1016/j.ijfoodmicro.2020.108884.
Hernández, F., V. García, J. Madrid, J. Orengo, P. Catalá, and M.D. Megías. “Effect of Formic Acid on Performance, Digestibility, Intestinal Histomorphology and Plasma Metabolite Levels of Broiler Chickens.” British Poultry Science 47, no. 1 (2006): 50–56. https://doi.org/10.1080/00071660500475574.
Hinton, M. “Antibacterial Activity of Short-Chain Fatty Acids.” The Veterinary Record 126 (n.d.): 416–21.
Hinton, M., and A. Linton. “Control of Salmonella Infections in Broiler Chickens by the Acid Treatment of Their Feed.” Veterinary Record 123, no. 16 (1988): 416–21. https://doi.org/10.1136/vr.123.16.416.
Humphrey, T. J., and D. G. Lanning. “The Vertical Transmission of Salmonellas and Formic Acid Treatment of Chicken Feed: A Possible Strategy for Control.” Epidemiology and Infection 100, no. 1 (1988): 43–49. https://doi.org/10.1017/s0950268800065547.
Khan, Rosina, Barira Islam, Mohd Akram, Shazi Shakil, Anis Ahmad Ahmad, S. Manazir Ali, Mashiatullah Siddiqui, and Asad Khan. “Antimicrobial Activity of Five Herbal Extracts against Multi Drug Resistant (MDR) Strains of Bacteria and Fungus of Clinical Origin.” Molecules 14, no. 2 (2009): 586–97. https://doi.org/10.3390/molecules14020586.
Koutsoumanis, Kostas, Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Alessandra De Cesare, et al. “Salmonella Control in Poultry Flocks and Its Public Health Impact.” EFSA Journal 17, no. 2 (2019). https://doi.org/10.2903/j.efsa.2019.5596.
Maciel, Bianca Mendes, Rachel Passos Rezende, and Nammalwar Sriranganathan. “Salmonella Enterica: Latency.” Current Topics in Salmonella and Salmonellosis, 2017. https://doi.org/10.5772/67173.
McHan, Frank, and Emmett B. Shotts. “Effect of Feeding Selected Short-Chain Fatty Acids on the in Vivo Attachment of Salmonella Typhimurium in Chick Ceca.” Avian Diseases 36, no. 1 (1992): 139. https://doi.org/10.2307/1591728.
Mkangara, M. and M., R. Mwakapuja, J. Chilongola, P. Ndakidemi, E. Mbega, and M. Chacha. “Mechanisms for Salmonella Infection and Potential Management Options in Chicken.” The Journal of Animal & Plant Sciences 30, no. 2 (April 2, 2020): 259–79. https://doi.org/10.36899/japs.2020.2.0050.
Mroz, Z., S.-J. Koopmans, A. Bannink, K. Partanen, W. Krasucki, M. Øverland, and S. Radcliffe. “Chapter 4 Carboxylic Acids as Bioregulators and Gut Growth Promoters in Nonruminants.” Biology of Growing Animals, 2006, 81–133. https://doi.org/10.1016/s1877-1823(09)70091-8.
OHMOMO, Sadahiro, Osamu TANAKA, Hiroko K. KITAMOTO, and Yimin CAI. “Silage and Microbial Performance, Old Story but New Problems.” Japan Agricultural Research Quarterly: JARQ 36, no. 2 (2002): 59–71. https://doi.org/10.6090/jarq.36.59.
Ranieri, Matthew L., Chunlei Shi, Andrea I. Moreno Switt, Henk C. den Bakker, and Martin Wiedmann. “Comparison of Typing Methods with a New Procedure Based on Sequence Characterization for Salmonella Serovar Prediction.” Journal of Clinical Microbiology 51, no. 6 (2013): 1786–97. https://doi.org/10.1128/jcm.03201-12.
Russell, J.B. “Another Explanation for the Toxicity of Fermentation Acids at Low Ph: Anion Accumulation versus Uncoupling.” Journal of Applied Bacteriology 73, no. 5 (1992): 363–70. https://doi.org/10.1111/j.1365-2672.1992.tb04990.x.
Thompson, J. L., and M. Hinton. “Antibacterial Activity of Formic and Propionic Acids in the Diet of Hens on Salmonellas in the Crop.” British Poultry Science 38, no. 1 (1997): 59–65. https://doi.org/10.1080/00071669708417941.
USDA – United States Department of Agriculture – Research, Education & Economics Information System. University of Georgia, 2015. https://portal.nifa.usda.gov/web/crisprojectpages/0228031-effect-of-fat-content-on-the-survival-of-salmonella-in-food.html.
“USDA Launches New Effort to Reduce Salmonella Illnesses Linked to Poultry.” USDA, October 19, 2021. https://www.usda.gov/media/press-releases/2021/10/19/usda-launches-new-effort-reduce-salmonella-illnesses-linked-poultry.
Van Immerseel, F., J. B. Russell, M. D. Flythe, I. Gantois, L. Timbermont, F. Pasmans, F. Haesebrouck, and R. Ducatelle. “The Use of Organic Acids to Combatsalmonellain Poultry: A Mechanistic Explanation of the Efficacy.” Avian Pathology 35, no. 3 (2006): 182–88. https://doi.org/10.1080/03079450600711045.
Van Immerseel, Filip, Jeroen De Buck, Isabel De Smet, Frank Pasmans, Freddy Haesebrouck, and Richard Ducatelle. “Interactions of Butyric Acid– and Acetic Acid–Treated Salmonella with Chicken Primary Cecal Epithelial Cells in Vitro.” Avian Diseases 48, no. 2 (2004): 384–91. https://doi.org/10.1637/7094.
Visscher, C. “Über Das Futter Helfen – Den Salmonellen Das Leben Schwer Machen.” Bauernblatt Schleswig-Holstein + Hamburg 68/164, no. 51 (December 20, 2014): 66–68.
Wernicki, Andrzej, Anna Nowaczek, and Renata Urban-Chmiel. “Bacteriophage Therapy to Combat Bacterial Infections in Poultry.” Virology Journal 14, no. 1 (September 16, 2017). https://doi.org/10.1186/s12985-017-0849-7.
Wessels, Kirsten, Diane Rip, and Pieter Gouws. “Salmonella in Chicken Meat: Consumption, Outbreaks, Characteristics, Current Control Methods and the Potential of Bacteriophage Use.” Foods 10, no. 8 (2021): 1742. https://doi.org/10.3390/foods10081742.

By Technical Team, EW Nutrition
Contamination with multiple mycotoxins is the rule for animal feeds, rather than the exception. Trial data shows that producers can prevent negative effects on animal health and performance by using high-performing toxin binders.

Mycotoxins pose an exceptional challenge for feed and animal producers. Generated by common molds, they occur in a great variety and numbers. Difficult to diagnose, mycotoxicosis in farm animals shows in a range of acute and chronic symptoms: decreased performance, feed refusal, poor feed conversion, reduced body weight gain, immune suppression, reproductive disorders, and residues in animal food products.
Regulatory thresholds for permissible mycotoxin levels in feed are derived from toxicological data on the effects of exposure of a certain species, at a certain production stage, to a single mycotoxin. This makes practical sense: while aflatoxins are carcinogens, fumonisins attack the pulmonary system in swine, for example. Mycotoxins also affect poultry in a different way than cattle, and broilers in a different way than breeders or laying hens, to mention more cases.
The problem is that, in reality, individual mycotoxin challenges are the exception. Animal diets are usually contaminated by multiple mycotoxins at the same time (Monbaliu et al., 2010; Pierron et al., 2016). Since 2014, EW Nutrition has conducted more than 50,000 mycotoxin tests on both raw material and finished feeds samples, across the globe. 85% of these samples were contaminated with more than one mycotoxin and one third positive for four or more mycotoxins.
The concurrent appearance of mycotoxins in feed can be explained as follows: each mold species has the capacity to produce several mycotoxins simultaneously. Each species, in turn, may infest several raw materials, leaving behind one or more toxic residue. In the end, a complete diet is made up of various raw materials with individual mycotoxin loads, resulting in a multitude of toxic challenges for the animals.
If animals were exposed to only one mycotoxin at a time, following the regulatory guidelines on maximum challenge levels would usually be enough to keep them safe. However, several studies have shown that the effects of exposure to multiple mycotoxins can differ greatly from the effects observed in animals exposed to a single mycotoxin (Alassane-Kpembi et al., 2015 & 2017). The simultaneous presence of mycotoxins may be more toxic than one would predict based on the known effects of the individual mycotoxins involved. This is because mycotoxins interact with each other. The interactions can be classified into three main different categories: antagonistic, additive, and synergistic (Grenier and Oswald, 2011).

Most of the mycotoxin mixtures lead to additive or synergistic effects. The actual consequences for the animal will depend on its species, age, sex, nutritional status, the dose and duration of exposure as well as environmental factors. What is clear is that mycotoxin interactions pose a significant threat to animal health and critically impede risk assessment.
Given their complex interactions, the toxicity of combinations of mycotoxins cannot merely be predicted based upon their individual toxicities. Mycotoxin risk assessments have to consider that even low levels of mycotoxin combinations can harm animal productivity, health, and welfare. Feed and animal producers need to be aware of which raw materials are likely to be contaminated with which mycotoxins, be able to accurately link them to the risk they pose for the animal and consequently take actions before the problems appear in the field.
Toxin binders that are effective against a broad spectrum of mycotoxins significantly reduce the risks of mycotoxin exposure. In vitro trial data shows that EW Nutrition’s cost-effective toxin-mitigating product Solis Max shows a high mitigation capacity, even at low inclusion rates (Figure 1). Importantly, Solis Max helps to reduce various mycotoxins’ negative effects on performance without any negative effects on nutrient absorption.
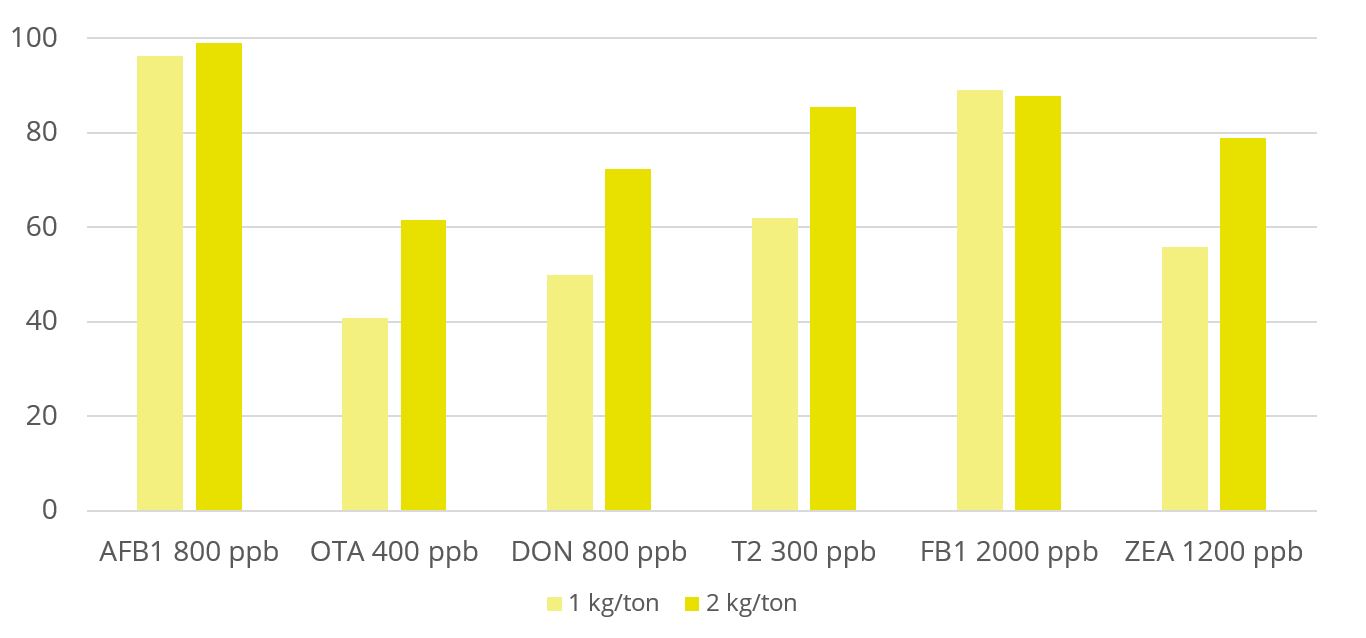
In another trial with 480 Ross 308 broilers, Solis Max 2.0 has demonstrated its ability to support animals coping with multiple mycotoxin challenges. For broilers challenged with 30 ppb AFB1 and 500 ppb OTA, Solis Max 2.0 supplementation resulted in a significantly 16% higher weight gain, an 18.5% higher final weight, and a 22% better FCR than the challenged group (Figure 2), which means a total recovery of the performance when compared with the non-challenged control.
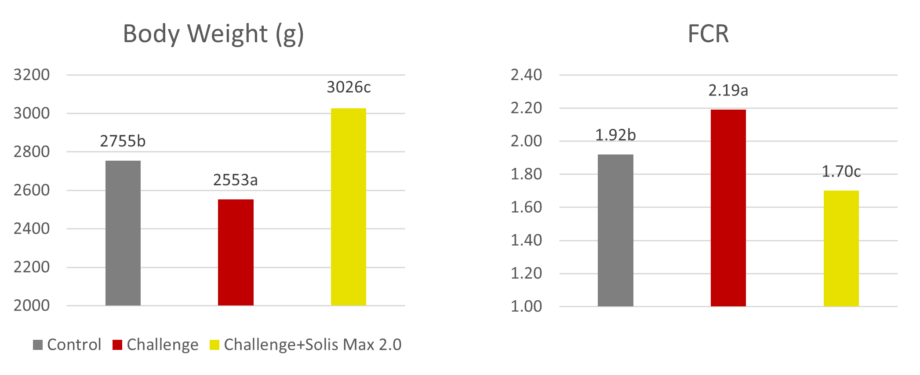
Liver health also improved: after 42 days of mycotoxin exposure, broilers receiving Solis Max 2.0 showed lower AST (-13%) and ALT (-44%) levels compared to the challenged group.
Mycotoxins interactions are the norm, not the exception. Yet, regulatory standards currently only cover the effects of individual mycotoxins, leaving productions exposed to risks of additive and synergistic mycotoxin interactions animals’ health and performance. Luckily, management options are available: Careful risk evaluation explicitly includes the threat of multiple contaminations. And producers can proactively ensure better health, welfare and productivity of their animals by investing in the right toxin mitigation solution for their business.
Alassane-Kpembi, Imourana, Olivier Puel, and Isabelle P. Oswald. “Toxicological Interactions between the Mycotoxins Deoxynivalenol, Nivalenol and Their Acetylated Derivatives in Intestinal Epithelial Cells.” Archives of Toxicology 89, no. 8 (August 2015): 1337–46. https://doi.org/10.1007/s00204-014-1309-4.
Alassane-Kpembi, Imourana, Gerd Schatzmayr, Ionelia Taranu, Daniela Marin, Olivier Puel, and Isabelle Paule Oswald. “Mycotoxins Co-Contamination: Methodological Aspects and Biological Relevance of Combined Toxicity Studies.” Critical Reviews in Food Science and Nutrition 57, no. 16 (November 2017): 3489–3507. https://doi.org/10.1080/10408398.2016.1140632.
Bensassi, Fatma; Gallerne, Cindy; Sharaf el dein, Ossama; Rabeh Hajlaoui, Mohammed; Lemaire, Christophe and Bacha, Hassen. “In vitro investigation of toxicological interactions between the fusariotoxins deoxynivalenol and zearalenone” Toxicon 84 (2014): 1-6. https://doi.org/10.1016/j.toxicon.2014.03.005.
Grenier, B., and I. Oswald. “Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions.” World Mycotoxin Journal 4, no. 3 (May 5, 2011): 285–313. https://doi.org/10.3920/wmj2011.1281.
Miazzo, R., M.F. Peralta, C. Magnoli, M. Salvano, S. Ferrero, S.M. Chiacchiera, E.C.Q. Carvalho, C.A.R. Rosa, and A. Dalcero. “Efficacy of Sodium Bentonite as a Detoxifier of Broiler Feed Contaminated with Aflatoxin and Fumonisin.” Poultry Science 84, no. 1 (January 2005): 1–8. https://doi.org/10.1093/ps/84.1.1.
Monbaliu, Sofie, Christof Van Poucke, Christ’l Detavernier, Frédéric Dumoulin, Mario Van De Velde, Elke Schoeters, Stefaan Van Dyck, Olga Averkieva, Carlos Van Peteghem, and Sarah De Saeger. “Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method.” Journal of Agricultural and Food Chemistry 58, no. 1 (2010): 66–71. https://doi.org/10.1021/jf903859z.
Pierron, Alix, Imourana Alassane-Kpembi, and Isabelle P. Oswald. “Impact of Mycotoxin on Immune Response and Consequences for Pig Health.” Animal Nutrition 2, no. 2 (2016): 63–68. https://doi.org/10.1016/j.aninu.2016.03.001.

By Dr. Inge Heinzl, Editor, EW Nutrition
In June 2017, the European Commission decided to ban the use of veterinary drugs containing high doses of zinc oxide (3000mg/kg) from 2022. The use of zinc oxide in pig production must then be limited to a maximum level of 150ppm. Companies have been on the lookout for effective alternative strategies to maintain high profitability.
Modern pig production is characterised by its high intensity. In many European countries, piglets are weaned after 3-4 weeks, before their physiological systems are fully developed (e.g. immune and enzyme system). Weaning and thus separation from the mother, as well as a new environment with new germs, means stress for the piglets. Besides, the highly digestible sow’s milk, for which the piglets are wholly adapted, is replaced by solid starter feed.
This, associated with the above-mentioned stressors, can result in reduced feed intake during the first week after weaning and therefore in a delayed adaptation of the intestinal flora to the feed. Since the immune system of animals is not yet fully functional, pathogens such as enterotoxic E. coli can colonize the intestinal mucosa. This can possibly develop into a dangerous dysbiosis, leading to an increased incidence of diarrhea. Inadequate absorption results in suboptimal growth with worse feed conversion. The consequences are economic losses due to higher treatment costs, lower yields, and animal losses.
Diarrhea is one of the most common causes of economic losses in pig production. In the past, this was the reason antibiotics were prophylactically used as growth promoters. Antibiotics reduce antimicrobial pressure and have an anti-inflammatory effect. In addition to reducing the incidence of disease, they eliminate competitors for nutrients in the gut and thus improve feed conversion.
However, the use of antibiotics as growth promoters has been banned in the EU since 2006 due to increased antimicrobial resistance. As a result, zinc oxide (ZnO) appeared on the scene. A study carried out in Spain in 2012 (Moreno, 2012) showed that 57% of piglets received ZnO before weaning and 73% during the growth phase (27-75 days).
What made the use of zinc oxide so attractive? Zinc oxide is inexpensive, available in many EU countries, and as a trace element it can be used in high doses through premixing. In some countries, however, a veterinary prescription is needed; in others, the use is already banned.
Zinc is a trace element involved in cell division and differentiation, and it influences the efficacy of enzymes. Since defence cells also need zinc, a supplementation that covers the demand for zinc strengthens the body’s defences. Through a positive effect on the structure of the gut mucosa membrane, zinc protects the body against the penetration of pathogenic germs.
If ZnO is used in pharmacological doses, it has a bactericidal effect against e.g. staphylococci (Ann et al., 2014) and various types of E. coli (Vahjen et al., 2016). Thus, prophylactic use prevents the incidence of diarrhea and the consequent decrease in performance. But the use of zinc oxide also has “side effects”.
Zinc belongs to the chemical group of heavy metals. For the use as a performance enhancer, it has to be administered in relatively high doses (2000–4000ppm). These high amounts are far above the physiological needs of the animals. With relatively low absorption rates (the bioavailability amounts to approximately 20% (European Commission, 2003)) and subsequent accumulation in manure, zinc can cause substantial contamination of the environment.
In addition to the accumulation of zinc in the environment, another aspect also plays an important role: according to Vahjen et al. (2015), a dose of ≥2500mg/kg of food increases the presence of tetracycline and sulfonamide resistance genes in bacteria. In the case of Staphylococcus aureus, the development of resistance to zinc is combined with the development of resistance to methicillin (MRSA; Cavaco et al., 2011; Slifierz et al., 2015). A similar effect can be observed in the development of multiresistant E. coli (Bednorz et al., 2013; Ciesinski et al., 2018). The reason for this is that the genes that encode antibiotic resistance, i.e. the ones that are “responsible” for the resistance, are found in the same plasmid (a DNA molecule that is small and independent of the bacterial chromosome).
The negative effects on the environment and the promotion of antibiotic resistance led to the European Commission’s decision in 2017 to completely ban zinc oxide as a therapeutic agent and as a growth promoter in piglets within five years.
By the 2022 deadline, the EU pig industry must find a solution to replace ZnO. It must develop strategies that make future pig production efficient, even without substances such as antibiotics and zinc oxide. To this end, measures should be taken at different levels, such as farm management and biosecurity (e.g. effective hygiene management). The promotion of intestinal health for high animal performance is most important, however.
The term eubiosis denotes the balance of microorganisms living in a healthy intestine, which must be maintained to prevent diarrhea and ensure performance. However, weaning, food switching, and other external stressors can endanger this balance. As a result, potentially pathogenic germs can “overgrow” the commensal microbiome and develop dysbiosis. Through the use of functional supplements, intestinal health can be improved.
Phytomolecules, or secondary plant compounds, are substances formed by plants with a wide variety of properties. The best-known groups are probably essential oils, but there are also bitter substances, spicy substances, and other groups.
In animal nutrition, phytomolecules such as carvacrol, cinnamon aldehyde, and capsaicin can help improve intestinal health and digestion. They stabilize the intestinal flora by slowing or stopping the growth of pathogens that can cause disease. How? Phytomolecules, for example, make the cell walls of several bacteria permeable so that cell contents can leak. They also partially interfere with the enzymatic metabolism of the cell or intervene with the transport of ions, reducing the proton motive force. These effects depend on the dose: all these actions can destroy bacteria or at least prevent their proliferation.
Another point of attack for phytomolecules is the communication between microorganisms (quorum sensing). Phytomolecules can prevent microorganisms from releasing substances known as autoinducers, which they need to coordinate joint actions such as the formation of biofilms or the expression of virulence factors.
Medium-chain triglycerides (MCT) and fatty acids (MCFA) are characterised by a length of six to twelve carbon atoms. Thanks to their efficient absorption and metabolism, they can be optimally used as an energy source in piglet feeding. MCTs can be completely absorbed by the epithelial cells of the intestinal mucosa and hydrolysed with microsomal lipases. Hence they serve as an immediately available energy source and can improve the epithelial structure of the intestinal mucosa (Hanczakowska, 2017).
In addition, these supplements have a positive influence on the composition of the intestinal flora. Their ability to penetrate bacteria through semi-permeable membranes and destroy bacterial structures inhibits the development of pathogens such as salmonella and coliforms (Boyen et al., 2008; Hanczakowska, 2017; Zentek et al., 2011). MCFAs and MCTs can also be used very effectively against gram-positive bacteria such as streptococci, staphylococci, and clostridia (Shilling et al., 2013; Zentek et al., 2011).
Prebiotics are short-chain carbohydrates that are indigestible for the host animal. However, certain beneficial microorganisms such as lactobacilli and bifidobacteria can use these substances as substrates. By selectively stimulating the growth of these bacteria, eubiosis is promoted (Ehrlinger, 2007). In pigs, mannan-oligosaccharides (MOS), fructooligosaccharides (FOS), inulin and lignocellulose are mainly used.
Another element of prebiotics’ positive effect on intestinal health is their ability to agglutinate pathogens. Pathogenic bacteria and MOS can bind to each other through lectin. This agglutination prevents pathogenic bacteria from adhering to the wall of the intestinal mucosa and thus from colonizing the intestine (Oyofo et al., 1989).
Probiotics can be used to regenerate an unbalanced gut flora. To do this, useful bacteria such as bifido or lactic acid bacteria are added to the food. They must settle in the gut and compete with the harmful bacteria.
There are also probiotics which target the communication between pathogens. In an experiment, Kim et al. (2017) found that the addition of probiotics that interfere with quorum sensing can significantly improve the microflora in weaned piglets and thus their intestinal health.
Organic acids show strong antibacterial activity in animals. In their undissociated form, the acids can penetrate bacteria. Inside, the acid molecule breaks down into a proton (H+) and an anion (HCOO-). The proton reduces the pH value in the bacterial cell and the anion interferes with the bacteria’s protein metabolism. As a result, bacterial growth and virulence are inhibited.
Today there are several possibilities in piglet nutrition to effectively support the young animals after weaning. The main objective is to maintain a balanced intestinal flora and therefore to sustain intestinal health – its deterioration often leads to diarrhea and hence to reduced returns. Intestinal health is promoted by stimulating beneficial bacteria and by inhibiting pathogenic ones. This can be achieved through feed additives that have an antibacterial effect and/or support the intestinal mucosa, such as phytomolecules, prebiotics, and medium-chain fatty acids. Through a combination of these possibilities, additive effects can be achieved. Piglets receive optimal support and the use of zinc oxide can be reduced.
Ann, Ling Chuo, Shahrom Mahmud, Siti Khadijah Mohd Bakhori, Amna Sirelkhatim, Dasmawati Mohamad, Habsah Hasan, Azman Seeni, and Rosliza Abdul Rahman. “Antibacterial Responses of Zinc Oxide Structures against Staphylococcus Aureus, Pseudomonas Aeruginosa and Streptococcus Pyogenes.” Ceramics International 40, no. 2 (March 2014): 2993–3001. https://doi.org/10.1016/j.ceramint.2013.10.008.
Bednorz, Carmen, Kathrin Oelgeschläger, Bianca Kinnemann, Susanne Hartmann, Konrad Neumann, Robert Pieper, Astrid Bethe, et al. “The Broader Context of Antibiotic Resistance: Zinc Feed Supplementation of Piglets Increases the Proportion of Multi-Resistant Escherichia Coli in Vivo.” International Journal of Medical Microbiology 303, no. 6-7 (August 2013): 396–403. https://doi.org/10.1016/j.ijmm.2013.06.004.
Boyen, F., F. Haesebrouck, A. Vanparys, J. Volf, M. Mahu, F. Van Immerseel, I. Rychlik, J. Dewulf, R. Ducatelle, and F. Pasmans. “Coated Fatty Acids Alter Virulence Properties of Salmonella Typhimurium and Decrease Intestinal Colonization of Pigs.” Veterinary Microbiology 132, no. 3-4 (December 10, 2008): 319–27. https://doi.org/10.1016/j.vetmic.2008.05.008.
Cavaco, Lina M., Henrik Hasman, Frank M. Aarestrup, Members Of Mrsa-Cg: Jaap A. Wagenaar, Haitske Graveland, Kees Veldman, et al. “Zinc Resistance of Staphylococcus Aureus of Animal Origin Is Strongly Associated with Methicillin Resistance.” Veterinary Microbiology 150, no. 3-4 (June 2, 2011): 344–48. https://doi.org/10.1016/j.vetmic.2011.02.014.
Ciesinski, Lisa, Sebastian Guenther, Robert Pieper, Martin Kalisch, Carmen Bednorz, and Lothar H. Wieler. “High Dietary Zinc Feeding Promotes Persistence of Multi-Resistant E. Coli in the Swine Gut.” Plos One 13, no. 1 (January 26, 2018). https://doi.org/10.1371/journal.pone.0191660.
Crespo-Piazuelo, Daniel, Jordi Estellé, Manuel Revilla, Lourdes Criado-Mesas, Yuliaxis Ramayo-Caldas, Cristina Óvilo, Ana I. Fernández, Maria Ballester, and Josep M. Folch. “Characterization of Bacterial Microbiota Compositions along the Intestinal Tract in Pigs and Their Interactions and Functions.” Scientific Reports 8, no. 1 (August 24, 2018). https://doi.org/10.1038/s41598-018-30932-6.
Ehrlinger, Miriam. 2007. “Phytogene Zusatzstoffe in der Tierernährung.“ PhD Diss., LMU München. URN: urn:nbn:de:bvb:19-68242.
European Commission. 2003. “Opinion of the Scientific Committee for Animal Nutrition on the use of zinc in feedingstuffs.” https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out120.pdf
Hanczakowska, Ewa. ”The use of medium chain fatty acids in piglet feeding – a review.” Annals of Animal Science 17, no. 4 (October 27, 2017): 967-977. https://doi.org/10.1515/aoas-2016-0099.
Hansche, Bianca Franziska. 2014. „Untersuchung der Effekte von Enterococcus faecium (probiotischer Stamm NCIMB 10415) und Zink auf die angeborene Immunantwort im Schwein. Dr. rer. Nat. Diss., Freie Universität Berlin. https://doi.org/10.17169/refubium-8548
Kim, Jonggun, Jaepil Kim, Younghoon Kim, Sangnam Oh, Minho Song, Jee Hwan Choe, Kwang-Youn Whang, Kwang Hyun Kim, and Sejong Oh. “Influences of Quorum-Quenching Probiotic Bacteria on the Gut Microbial Community and Immune Function in Weaning Pigs.” Animal Science Journal 89, no. 2 (November 20, 2017): 412–22. https://doi.org/10.1111/asj.12954.
Oyofo, Buhari A., John R. Deloach, Donald E. Corrier, James O. Norman, Richard L. Ziprin, and Hilton H. Mollenhauer. “Effect of Carbohydrates on Salmonella Typhimurium Colonization in Broiler Chickens.” Avian Diseases 33, no. 3 (1989): 531–34. https://doi.org/10.2307/1591117.
Shilling, Michael, Laurie Matt, Evelyn Rubin, Mark Paul Visitacion, Nairmeen A. Haller, Scott F. Grey, and Christopher J. Woolverton. “Antimicrobial Effects of Virgin Coconut Oil and Its Medium-Chain Fatty Acids On Clostridium Difficile.” Journal of Medicinal Food 16, no. 12 (December 2013): 1079–85. https://doi.org/10.1089/jmf.2012.0303.
Slifierz, M. J., R. Friendship, and J. S. Weese. “Zinc Oxide Therapy Increases Prevalence and Persistence of Methicillin-Resistant Staphylococcus Aureus in Pigs: A Randomized Controlled Trial.” Zoonoses and Public Health 62, no. 4 (September 11, 2014): 301–8. https://doi.org/10.1111/zph.12150.
Vahjen, Wilfried, Dominika Pietruszyńska, Ingo C. Starke, and Jürgen Zentek. “High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs.” Gut Pathogens 7, article number 23 (August 26, 2015). https://doi.org/10.1186/s13099-015-0071-3.
Vahjen, Wilfried, Agathe Roméo, and Jürgen Zentek. “Impact of zinc oxide on the immediate postweaning colonization of enterobacteria in pigs.” Journal of Animal Science 94, supplement 3 (September 1, 2016): 359-363. https://doi.org/10.2527/jas.2015-9795.
Zentek, J., S. Buchheit-Renko, F. Ferrara, W. Vahjen, A.G. Van Kessel, and R. Pieper. “Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets” Animal Health Research Reviews 12, no. 1 (June 2011): 83-93. https://doi.org/10.1017/s1466252311000089.

By Dr. Inge Heinzl, Editor, EW Nutrition
According to the American Medical Association, antimicrobial resistance is one of the main threats to public health nowadays. More than 2 million people are infected with bacteria resistant to different types of antibiotics every year (Marquardt and Suzhen, 2018). Prof Dame Sally Davies (2012), Chief Medical Officer for England, mentions that antibiotics are losing their effectiveness at alarming rates. Bacteria are finding ways to survive the antibiotics, so these molecules no longer work. O’Neill (2016) predicted in his report that 10 million people a year could be dying by 2050 due to antimicrobial resistance.
Antimicrobial resistance is a natural process but this is accelerated by inappropriate prescribing of antimicrobials, poor infection control practices and the unnecessary use of antimicrobials in agriculture (Barber and Sutherland, 2017).
Resistance to specific antibiotics occurs through mutations that enable the bacteria to withstand an antibiotic treatment. One mechanism is the production of enzymes degrading or altering the antibiotic, rendering them harmless. The elimination of entrances for antibiotics or the development of pumps discharging them is another possibility. A further option is the elimination of the targets the antibiotic would attack.
So-called “resistance genes” are responsible for resistance. These genes can be transferred from one bacterium to another and also from beneficial bacteria to harmful ones. When antibiotics are used, “normal” bacteria are killed; the resistant ones survive and have all possibilities to proliferate. The Dutch Government has been tracking resistant bacteria in poultry flocks for the last two decades. A clear correlation between antibiotic use and the percentage of resistance could be observed. The good thing: according to the 2020 MARAN report (De Greeff et al., 2020), by reducing the use of antibiotics, the occurrence of resistances can be pushed back.
Figure 1. Sales of antibiotics from 1999 to 2016 and the development of resistances (MARAN report, 2018)
In pig production, antibiotics are often used in stressful situations such as weaning or moving. Antibiotics decrease the pathogenic pressure in animals and help them overcome these critical periods. Disadvantage: Antibiotics do not differentiate between good and bad but between susceptible and resistant. Therefore, also the beneficial gut flora gets destroyed through antibiotic treatment, and resistance is spread.
After the ban of antibiotic growth promoters in Europe in 2006, the US has also made considerable efforts to reduce the use of antibiotics.
When antibiotics are taken out of livestock production, measures in different areas must be implemented to keep performance and profitability high. Without supporting the animals by other means, they will get sick and even die in acute cases. Subclinical disease forms reduce their feed intake, and growth performance consequently decreases. According to literature, losses due to decreased average weight gain can be up to $40 per pig (Hao et al., 2014).
To support pigs, especially during the afore-mentioned critical periods, alternatives focusing on the maintenance of gut health and, therefore, also overall health must be chosen. This goal can only be achieved by balancing the intestinal flora with reducing pathogenic bacteria occurrence.
Phytomolecules are produced by plants to defend themselves against predators or pathogens. Farmers use the substances in animal feeds to support digestion, improve palatability, but also to reduce pathogenic pressure (Baser and Buchbauer, 2010).
In animal feeding, different application forms are available:
A trial conducted at the Federal University of Lavras (Brazil) evaluated if phytomolecules as a regular diet component can deliver the same effects on growth performance as AGPs in pig production.
For the trial, 108 castrated newborn male pigs were allocated to 3 groups (control, AGP (antibiotic growth promoters), and Activo). Pigs were weaned at 23 days of age with an average weight of 6.3 kg. They were fed a 3-phase diet (nursery, growing, and finishing). The inclusion rates of the additives (antibiotics and phytomolecules-based product – Activo) are shown in table 1.
On days 0, 1, and 2 of the experiment, the animals were challenged by applying a solution containing 107 CFU of E. coli K88, producing the toxins LT, Sta, and bST. Additionally, during the two last days before the growing phase, the animals were exposed to 5h of heat stress, using infrared lamps and closed windows. The parameters weight gain, final weight, FCR, and gut flora composition in the cecum were evaluated.
| Phase | Control | AGP | Activo | |
| Nursery | 0-7 days | — | Gentamycin 2.7kg/t | 0.4kg/t |
| 8-42 days | — | Haloquinol 0.2kg/t | 0.3kg/t | |
| Growing | 42-52 days | — | Tylosin 0.45kg/t | 0.4kg/t |
| 53-87 days | — | Enramycin 0.125kg/t | 0.2kg/t | |
| Finishing | 88-97 days | — | Tylosin 0.45kg/t | 0.4kg/t |
| 98-126 days | — | Enramycin 0.063kg/t | 0.2kg/t | |
Table 1. Inclusion rate of the additives in the feed
AGP: Antibiotic growth promoter; Activo: product based on phytomolecules, microencapsulated (EW Nutrition)
The results of this trial are shown in figure 2.
Concerning growth performance, the group fed the phytomolecules-based product Activo showed a 4.36 kg higher final weight after 126 days than the group provided AGPs (p=0.11), resulting in a 3.28 kg higher weight gain (p=0.21) and a 13 points better feed conversion.
Figure 2. Data of growth performance including final weight, weight gain and FCR adjusted to 100kg
The evaluation of some bacteria naturally occurring in the gut flora showed that, in contrast to the antibiotic prophylaxes, Activo has no negative impact on E. coli, Lactobacillus and Bifidobacterium. However, the antibiotic group showed a slight decrease in the population of Lactobacilli (Figure 3).
Figure 3. Impact of antibiotics and phytomolecules (Activo) on the composition of the gut flora
This trial shows Activo increasing growth performance and feed conversion without any negative impact on gut flora. The addition of phytomolecules (Activo) to the feed is documented as optimal long-term support instead of antibiotic growth promoters.
In a trial conducted in the USA, a product containing phytomolecules and organic acids (Activo Liquid, EW Nutrition) was compared to an antibiotic for controlling bacterial diseases in US pig production (Mecadox). For the trial, a total of 360 weanling pigs, about 19 days old and weighing 5.70 kg, were divided into four groups. Each group consists of 9 pens with 10 animals per pen. All groups were fed a 3-phase diet.
To the different trial groups, the following products were added (table 2):
| Feeding valid for all groups | Group / Product | Inclusion rate and period of application | |
| 3-phase feeding after weaning: | Mecadox | 50 g/t of feed during the whole period | |
| Phase I (days 0-7): | 23 % CP, 5.4 % CF | Activo Liquid 3 | 375 ml/1000 L of water for 3 days post-weaning |
| Phase II (days 8-21): | 21 % CP, 4.1 % CF | Activo Liquid 5 | 375 ml/1000 L of water for 5 days post-weaning |
| Phase III (days 22-42): | 19 % CP, 4.4 % CF | Activo Liquid 7 | 375 ml/1000 L of water for 7 days post-weaning |
These performance parameters were evaluated: live weight, daily gain, daily feed intake, feed:gain ratios, and mortality.
Table 2. Feeding and inclusion of the additives
The results of the trial are shown in figure 4. Concerning growth, no significant differences could be seen between the groups, only numerical differences. Live weight in the antibiotic group was 25.95 kg, and in the Activo Liquid groups, it ranges from 25.77 kg (shortest period of application) to 26.20 kg (see below). This resulted in calculated values for an average daily gain of 473 g in the Mecadox fed animals and 463 to 487g in the Activo Liquid groups. Due to a lower feed intake per kg of weight gain, all groups fed Activo Liquid showed a significantly (p=0.05) better feed conversion than the Mecadox group.
Figure 4. Live weight in the groups fed the antibiotic Mecadox and the phytomolecules-based product Activo Liquid for different periods
Average daily gain in the different trial groups
Average daily feed intake in the different trial groups (P=0.05)
Concerning mortality, the group fed Activo Liquid for 5 days showed the lowest mortality rate of 1.1% (figure 5).
Figure 5. Feed:gain ratio in the different trial groups (P=0.05) & Mortality rates
Considering all parameters, the group fed Activo Liquid for five days provided the best results: numerically the lowest mortality rate, highest daily gain, and one of the two lowest feed:gain ratios. This trial concludes that Activo Liquid with an application period of five days can safely replace antibiotic growth promoters in the diet. Therefore, Activo Liquid is an interesting tool to additionally support pigs during critical periods of life.
The trials conducted with two types of phytomolecules-based products show that phytomolecules efficiently support pigs to achieve their genetic potential. A basic supply of these substances within the feed yields results similar to those of animals receiving antibiotic growth promoters (AGPs). In challenging situations like weaning, additional short-term supply is recommended, which can be done with liquid products via the waterline.
With this strategy, the use of antibiotic growth promoters and, therefore, antibiotics in general can be drastically reduced. This approach can help decrease antimicrobial resistance and, not to be forgotten, accommodates final customers’ requests for the lower usage of antibiotics in livestock.
Barber, Sarah, and Nikki Sutherland. “O’Neill Review into Antibiotic Resistance.” House of Commons Library, March 6, 2017. https://commonslibrary.parliament.uk/research-briefings/cdp-2017-0074/.
Baser, Kemal Hüsnü Can, and Gerhard Buchbauer. Handbook of Essential Oils: Science, Technology, and Applications. Boca Raton, FL: Taylor & Francis distributor, 2010.
Davies, Dame Sally. “Antibiotic Resistance ‘Big Threat to Health’.” BBC News. BBC, November 16, 2012. https://www.bbc.co.uk/news/health-20354536.
De Greeff, S.C., A.F. Schoffelen, and C.M. Verduin. “MARAN Reports.” WUR. National Institute for Public Health and the Environment – Ministery of Health, Welfare and Sport, June 2020. https://www.wur.nl/en/Research-Results/Research-Institutes/Bioveterinary-Research/In-the-spotlight/Antibiotic-resistance/MARAN-reports.htm.
Hao, Haihong, Guyue Cheng, Zahid Iqbal, Xiaohui Ai, Hafiz I. Hussain, Lingli Huang, Menghong Dai, Yulian Wang, Zhenli Liu, and Zonghui Yuan. “Benefits and Risks of Antimicrobial Use in Food-Producing Animals.” Frontiers in Microbiology 5, no. Art. 288 (2014): 1–11. https://doi.org/10.3389/fmicb.2014.00288.
Marquardt, Ronald R, and Suzhen Li. “Antimicrobial Resistance in Livestock: Advances and Alternatives to Antibiotics.” Animal Frontiers 8, no. 2 (2018): 30–37. https://doi.org/10.1093/af/vfy001.
O’Neill, J. “Tackling Drug-Resistant Infections Globally.” Review on Antimicrobial Resistance. Wellcome Trust / HM Government, May 19, 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.


By Dr. Inge Heinzl, Editor, EW Nutrition
Diarrhea causes a higher workload, increased costs for treatment, losses, and, of course, lower benefits for the farmer. But not all diarrheas are equal. How do they differ, where do differences come from, and what can you do to protect your animals?

In general, diarrhea is characterized by more liquid being secreted than being resorbed. However, diarrhea is not a disease but only a symptom. Diarrhea has a protective function for the organism: the higher liquid volume in the gut increases motility, and pathogens and toxins are more readily excreted.
Diarrhea can occur for several reasons. It can result from inadequate nutrition but also the reaction to an infection by pathogens such as bacteria, viruses, and protozoa.
Depending on how the accumulation of fluid in the gut is generated, there are different kinds of diarrhea:
Due to multiple infections, diarrhea often is a mixture of different forms.
For the occurrence of diarrhea, different causers can be a possibility. Besides infectious pathogens, also the feed must be considered.
E. coli is a common agent of the gut microflora and in general it is harmless. However, E. coli can also be the cause of different types of diarrhea, depending on the virulence factors. Virulence factors of E.coli are, e.g., fimbria for the attachment to intestinal receptors or the ability to produce toxins influencing the secretion of ions and liquids. Example: enterotoxic E. coli (ETEC) F5 and F41 occurring during the first days of life.
In general, Salmonella plays a secondary role in calf diarrhea. Of the Salmonella serovars, mainly S. Typhimurium and S. Dublin are found in calves. Salmonella produces enterotoxins that attack the intestinal wall.
Clostridia infections belong to the most expensive ones in cattle farming globally. In herbivores, clostridia are part of the normal flora of the gastrointestinal tract; only a few types can cause severe disease. In calves, the necrotizing toxin-producing Clostridium perfringens can lead to enterotoxaemia manifesting in acute bloody diarrhea.
Rotavirus, which occurs mainly during the 5th -15th day of life, is the most common viral pathogen causing diarrhea in calves and lambs. If more enterocytes are destroyed than regenerated by the organism, the resorption surface in the gut decreases. With increasing age, animals develop immunity against this pathogen.
Coronavirus usually attacks calves at the age of 5 – 21 days (mainly correlated with the decreasing concentration of antibodies in maternal milk). They cause similar lesions in the intestine as rotavirus but additionally lead to necrosis of the crypts in the large intestine. The digestive and absorptive function is lost, resulting in reduced reabsorption of fluids. 3 to 20 % of diarrhea arising in calves is caused by Coronavirus.
Cryptosporidium parvum (mainly 1-2 weeks after birth) belongs to the coccidia and is presumed to be the most common pathogen to cause diarrhea (prevalence up to more than 60 %) in calves. Cryptosporidium is transmitted via oocysts found in feces and on the farm equipment. Cryptosporidia destroy the microvilli in the gut, the function of the gut mucosa is reduced, the resorption area decreases. Consequence: loss of enzyme activity and, therefore, an insufficient breakdown of sugar and protein, resulting in malabsorption.
In general, raw materials which cannot be well digested by the calf (mainly soya products, often used in milk replacers) or which cause allergy can cause diarrhea in calves. Also, antibiotics can lead to an imbalance of the intestinal flora, destruction of the villi, and malabsorptive diarrhea.
A field study with the egg powder-based product Globigen Dia Stop was conducted with 16 calves suffering from diarrhea. They were fed twice daily 50 g of Globigen Dia Stop stirred into the milk replacer.
Result (fig. 1): already one day after the first application of Globigen Dia Stop, 50 % of the calves recovered. After seven days, all calves overcame diarrhea. On average, one calf needed 2,4 treatments to show a full recovery from diarrhea (≙ 1,25 treatment days).
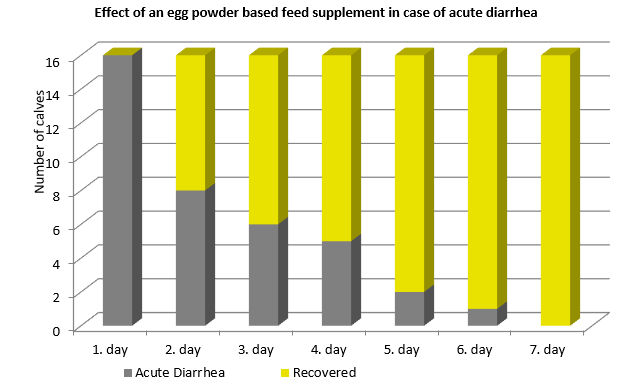
Egg immunoglobulins can effectively support calves in their fight against diarrhea. Immunoglobulins can act against bacteria, parasites, and viruses, not only against bacteria as antibiotics do. With egg immunoglobulin-based products, the farmer has a tool at his disposal that is easy to handle and does not require a withdrawal period. As there is no danger of the generation of resistance, these products are ideal for reducing the use of antibiotics in animal production.
Article initially published in NutriNews

By Lea Poppe, Technical Manager – Europe, EW Nutrition
Humans and animals protect themselves against diseases with specific antibodies (immunoglobulins). They receive antibodies from their mother or a vaccination (passive immunity) or produce them themselves after contact with a pathogen (active immunity). To be protected by a high passive immunity during the first weeks of life, a calf must receive high-quality colostrum with a sufficient amount of farm-specific antibodies as early as possible after birth.

In 2015, the Ludwig Maximilian University of Munich examined the immunoglobulin supply of 1,242 newborn calves. This study showed that more than half of the calves were undersupplied: 23% severely (<5mg IgG / ml blood serum) and 36% slightly undersupplied (5-10mg IgG/ml). The supply situation was only satisfactory in 41% of the calves (> 10 mg IgG/ml).
Undersupply results in higher susceptibility to disease, higher mortality, and lower daily weight gain. This entails increased rearing costs. Besides, only healthy calves can achieve their full potential as adult animals. For example, when a calf experiences even mild diarrhea, it is expected to produce 344 kg less milk the first lactation (Welsch, 2016). Possible causes of diarrhea are infectious factors such as viruses (rota, coronaviruses), bacteria (E. coli) and parasites (cryptosporidia), but also non-infectious factors such as poor husbandry and feeding errors.
In December 2020, EW Nutrition conducted a telephone survey among 55 dairy cattle consultants and veterinarians from Spain, Germany, France, Poland, and Great Britain to review calves’ passive immunity.
This survey confirmed that calves lack sufficient amounts of immunoglobulins: 69.1% of respondents thought that calves were undersupplied. 76.4% of them saw a clear connection between early-occurring diarrheal diseases and calves’ insufficient passive immunity. Respondents came to these conclusions even though more than half of them thought that colostrum quality had not deteriorated during the last years (56.4%).
Egg immunoglobulins offer one way to support calves in case of diarrhea. Chickens form antibodies (IgY from “Immunoglobulins in Yolk”) against all disease pathogens they encounter and release them into the egg as an immunological starting aid for the chick. It does not matter whether the disease is relevant to poultry or cattle.
These antibodies can be used to improve poor-quality colostrum or to support the calf during acute diarrhea. Studies show that egg immunoglobulins act in calves’ intestines, where they can bind and block diarrhea pathogens (Ikemori et al., 1992).
A feeding study with 39 female newborn calves took place on an 800-cow dairy farm in Brandenburg, Eastern Germany. The objective was to examine whether the IgY-containing complementary feed Globigen Colostrum effectively supports calves during the first critical period. For the experiment, all calves were given high-quality colostrum (4L within 2 hours after birth). During the first 5 days of life, the 19 calves in the test group additionally received 100g of the complimentary feed stirred into the colostrum (day 1) or the mixed colostrum (days 2 – 5).
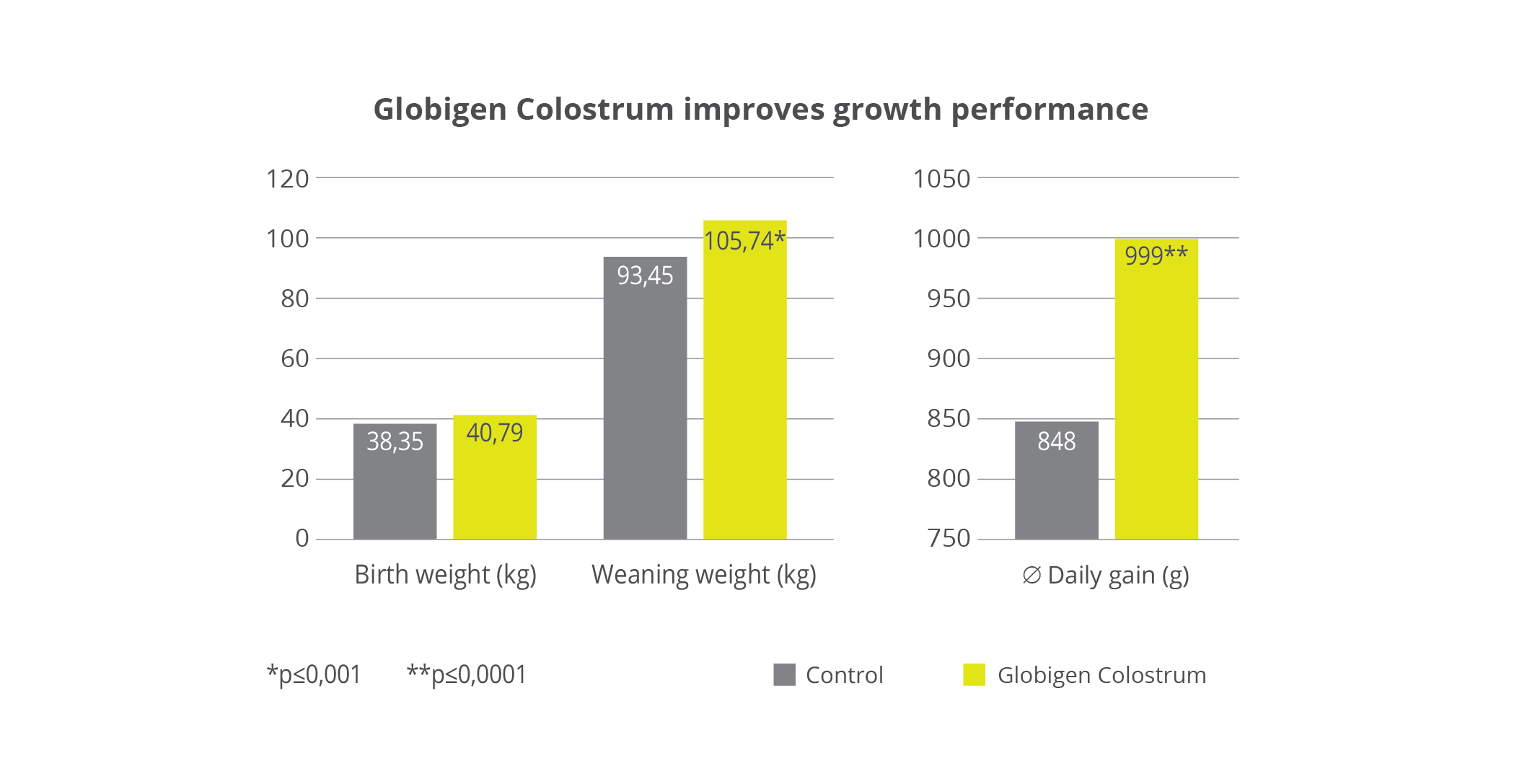
Result: The daily weight gain for the test group was 18% higher than in the control group (+ 151g). This resulted in 13% higher weaning weights (see above).
Three calves in the control group had mild diarrhea; in the test group, only one calf. However, antibiotics did not have to be used to treat the diarrhea.
The IgY-based product Globigen Calf Paste was tested on two dairy farms in Russia. These trials focused on reducing neonatal diarrhea, which occurs in the first 2 to 3 weeks of life. The product, a 30ml oral syringe with a dosing ring, was administered at a rate of 10ml per day for the first three days of life. On the first farm in the Belgorod region, the trial and control groups consisted of 11 calves each. On the 10th day of life, the diarrhea incidence per group was checked: while 73% of the calves in the control group had diarrhea, requiring antibiotics, only 1 calf of the trial group had diarrhea, and no antibiotic treatment was needed. On the second farm in the Moscow region, where the groups encompassed 20 calves each and observations took place on the 5th day of life, results were similar: 75% of the control animals suffered from diarrhea, but just 3 calves in the trial group showed signs of diarrhea.
In another trial, carried out with 38 calves on a dairy farm with 550 cows in North Rhine-Westphalia, Western Germany, the dietetic feed supplement Globigen Dia Stop was tested. This product is also based on egg immunoglobulins.
Only calves showing newborn diarrhea were used for this experiment. When diarrhea occurred, the 21 calves in the test group received 50g Globigen Dia Stop twice a day in addition to their milk drink. The diseased calves in the control group (17 calves) were given a rehydration solution, stirred into water, twice a day.
If the diarrhea could not be stopped after four days in the calves of either group, the animals were treated by a veterinarian.
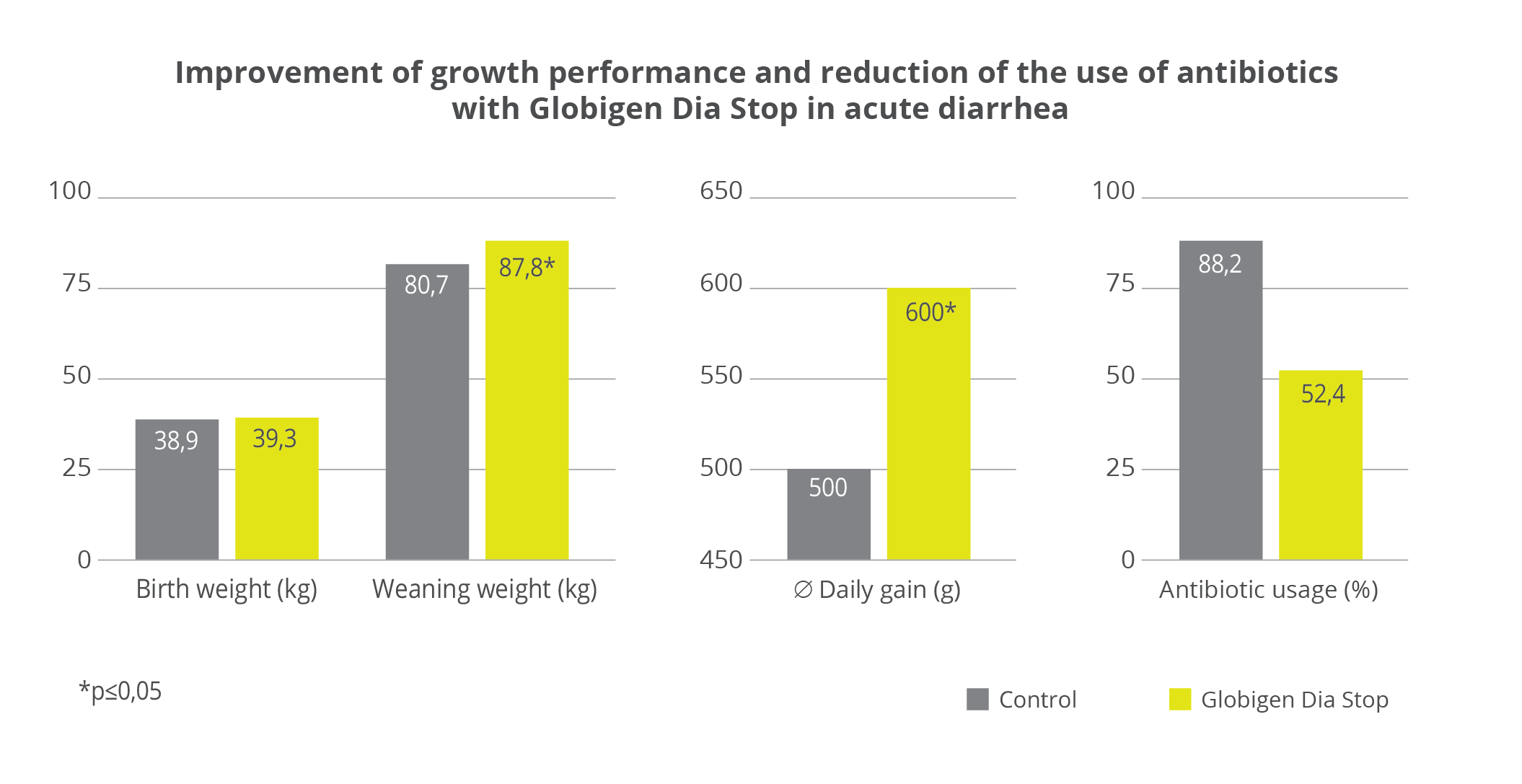
Result: In the test group, 100g (+ 20%) and thus significantly higher daily gains were achieved, which led to a 9% higher weaning weight. Furthermore, over 40% fewer calves had to be treated with antibiotics in the Globigen Dia Stop group than in the control group. (see above)
The results of these studies indicate that the administration of egg antibodies (IgY) to calves supports intestinal health and has a positive effect on calves’ performance. Globigen supplementation can likely reduce diarrhea incidence and severity, especially in the critical first phase of the calves’ life – thus ensuring high performance in the long term.