Xylanase solutions for broiler feed: Enzyme innovation finally hits the market

By Dr. Ajay Awati, Global Category Manager for Gut Health and Nutrition, EW Nutrition, and Dr. Howard Simmins, InSci Associates
After 30 years of stagnating solutions, in-feed xylanase innovation has finally arrived – with a complete focus on the needs of the broiler feed industry.

It has been over 30 years since xylanase was first introduced in broiler diets in Europe. In the meantime, it has been widely used worldwide with few, if any, major improvements. While the animal feed industry evolved in terms of production landscape, feed processing technologies and use of various by-products, xylanase enzyme technology did not keep pace. In fact, it did not evolve to meet customers’ changing needs and provide that much-needed flexibility of diet formulation for a commercial nutritionist. The wait is over: new in-feed xylanase technology is about to revolutionize broiler nutrition.
Why we need innovative xylanase enzymes for broiler production
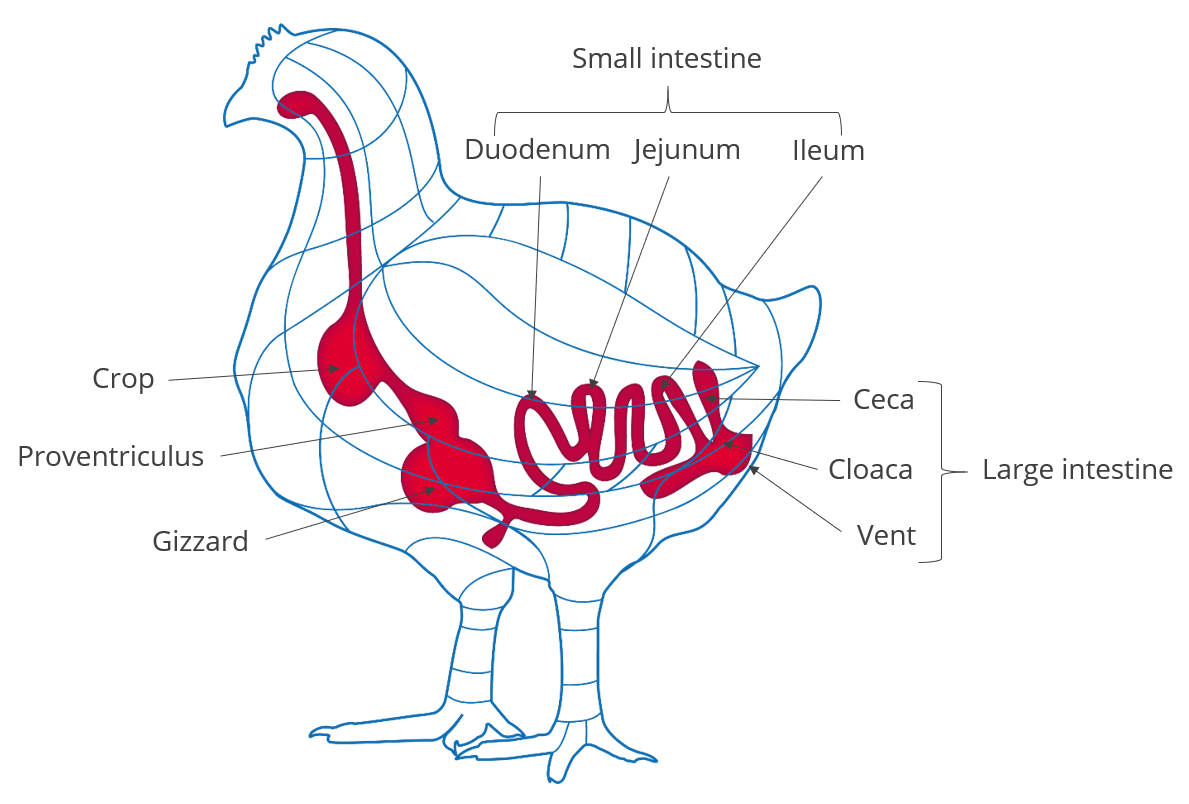
Initially, in the 1980s, xylanase was leveraged from industries unrelated to animal production into the feed business. Gut viscosity had been a continuing problem in broiler chickens fed wheat-based diets. It led to an increased risk of enteric disease, generally reducing performance. Xylanase was shown to reduce gut viscosity in wheat-based feed by breaking down soluble arabinoxylans.
As a result, the birds grew as well as if they were fed a low-viscosity corn/soya diet. An additional benefit was lower disease risks from the reduced level of anti-nutritional factors (ANFs) and the multiple negative effects of viscosity in the intestine.
In addition to reducing viscosity, xylanase augments the release in the small intestine of nutrients from previously undigested feedstuffs. The outcome has been the use of an energy matrix value for xylanase, which essentially helps diets through least-cost formulation.
These effects account for the growth of xylanase use in the monogastric feed market. Today, the penetration is above 50%.
Limitations of existing xylanase solutions
Leveraging xylanases from other industries for viscosity reduction in poultry comes with a couple of distinct limitations:
- Most broiler diets around the globe are based on a corn-soybean formulation, which contains far higher levels of insoluble arabinoxylans than soluble arabinoxylans. In such cases, viscosity is a relatively minor issue compared to the anti-nutritional effect of insoluble arabinoxylans.
- The reduction of gut viscosity is less relevant in other poultry sectors, such as laying hens and turkeys.
Commercial xylanases would be required to break down insoluble NSPs in which substrate activity may be limited and difficult to predict. Fiber constituents of different cereal grains used in feed are highly variable. By- and co-products derived from cereals contain even more complex fiber components, altered further by the manner of processing that the raw material has undergone.
Additionally, poultry response is highly variable: For an individual bird, the effectiveness of xylanase depends on the enzyme’s interaction with feed in the gastrointestinal tract (GIT) of the animal, which varies depending on the species and the animal’s age. This may explain why xylanase penetration on the feed market is not as high as that of phytase.
GH10: the next-level xylanase for feed application
A xylanase for feed is required to provide multiple functionalities, of which four are essential:
- Capacity to break down soluble and insoluble arabinoxylan across a range of typical feedstuffs
- Rapid activity at optimal pH in the preferred section of the GIT
- No inhibition in the presence of xylanase inhibitors
- Comprehensive feed processing thermostability
The GH11 family of xylanases commonly used in feed does not offer these aggregated benefits. They successfully reduce soluble NSPs in wheat-based diets, hence lowering the viscosity level in the broiler GIT. However, they are less effective in the presence of insoluble NSPs in which the arabinoxylan backbone is more complex.
Why GH10 instead of GH11?
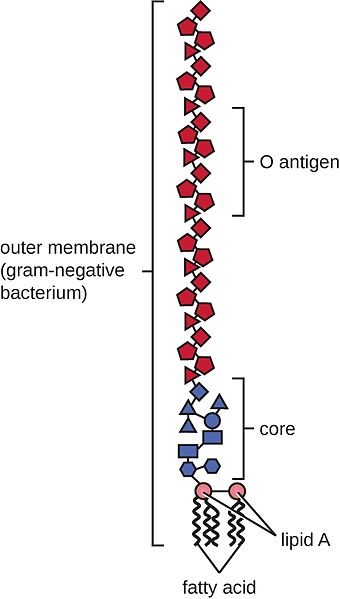
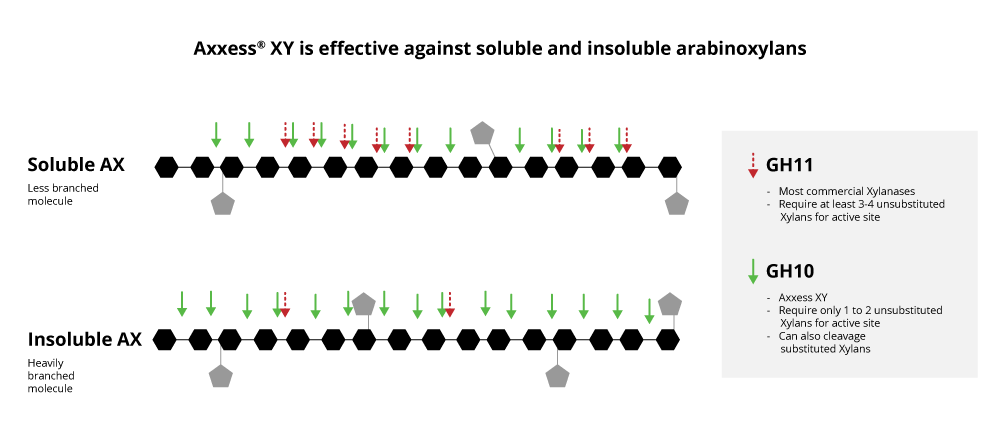
The explanation for this can be found in the 3-dimensional structure of the GH11 xylanase. The activity of GH11 xylanases requires 3 or 4 consecutive unsubstituted xylan monomers on the backbone to find an active site. That is why they are hindered by the presence of branches, or side chains, on arabinose backbones. Consequently, they are highly specific, favoring the particularly low-branching wheat backbone.
Xylanases from the GH10 family are entirely different. Although well known, they have not been used in feed yet. The GH10 xylanases require two or fewer consecutive unsubstituted xylan monomers on the backbone to find an active site. Therefore, they can act on xylose residues near branches. This results in both more and shorter xylo-oligomers than found with GH11 xylanases. In simple terms, the GH10 xylanases have a less deep cleft than the GH11 xylanases, providing greater catalytic versatility (Pollet 2010).
Significantly, this potentially allows a broader range of feedstuffs to be incorporated into the complete diet, including co- and by-products, while maintaining performance. Therefore, with GH10, higher levels of cheaper ingredients may be included, with a significant value proposition of further reducing feed costs.
GH10 xylanases generate a range of important prebiotics
As early as 1995, it was proposed that xylanase may affect microbial activity in the gastrointestinal tract through the provision of fermentable oligosaccharides and low molecular weight polysaccharides. These are produced from the hydrolysis of soluble and insoluble arabinoxylans in cereals.
A development of particular interest is that the GH10 xylanases break down the backbone of different fibre components into small xylooligosaccharides (XOS) and arabino-xylanoligosaccharides (AXOS). This action, research shows, has value in supporting the selective growth of fibre-degrading bacteria in the large intestine, conferring positive effects on the host’s health.
The most well-known probiotic strains belong to bifidobacteria and lactobacilli, which have quite different XOS and AXOS utilization systems. Bifidobacterium adolescentis has been shown to consume AXOS and XOS, whereas Lactobacillus brevis utilises only XOS. The outcome is that AXOS releases butyrate, the short-chain fatty acid, which can improve the host’s gut barrier function, as well as reduce Salmonella colonization in broilers. Alongside these health benefits, their presence may improve performance also by reducing FCR. (Courtin et al. 2008; Ribeiro et al. 2018)
As mentioned earlier, the GH10 xylanase requires only two consecutive unsubstituted xylan monomers to cleave the xylan main chain, whereas a GH11 xylanase requires 3 or 4 consecutive unsubstituted xylan monomers. Therefore, the number of potential AXOS and XOS oligomers is higher from the action of the GH10 xylanase. This results in a wider size range of oligomers. The range is valuable as the effect is spread across the large intestine, each oligomer having a different fermentation rate. Consequently, the large intestine’s microbial activity becomes saccharolytic, which potentially reduces the undesirable products of proteolytic degradation, such as phenols and cresols.
Prebiotic combinations will vary depending on the substrate available. However, there is more flexibility in breaking down insoluble NSPs across different feedstuffs using GH10 xylanase compared to GH-11 xylanase.
The future of xylanase: Reducing feed costs through flexible formulation
EW Nutrition’s GH10-based AXXESS XY xylanase, specifically developed for animal feed, has a wide-ranging activity across typical substrates, both in corn-soy and wheat-soy diets. It also allows for a greater proportion of cheaper ingredients, enabling increased flexibility in feedstuff choices and resulting in more stable feed pricing. The activity of the GH10 xylanase in producing oligomers from the breakdown of the arabinoxylan backbone also indicates that it can produce a greater number and diversity of valuable prebiotics that sustain the growth of fiber-degrading microbiota. Consequently, the metabolism of the large intestine is shifted from proteolytic to saccharolytic, which supports the animal’s general health.
The combination of these benefits from using this xylanase results in a bird with a balanced digestive system that is more robust in the face of environmental and health challenges, supporting better performance. Furthermore, this novel enzyme solution gives nutritionists a reliable tool to reduce feed costs by being flexible in diet formulation and opportunistic in using raw materials while maintaining consistency in animal performance. Especially in these times of supply problems and raw material price hikes, such advantages are invaluable.
The naturally thermostable AXXESS XY 1000G is the most advanced xylanase yet. It is a GH10 xylanase that delivers what the industry has been asking for: a fiber-degrading enzyme suited for all poultry feed.
References
Courtin, Christophe M, Katrien Swennen, Willem F Broekaert, Quirine Swennen, Johan Buyse, Eddy Decuypere, Christiaan W Michiels, Bart De Ketelaere, and Jan A Delcour. “Effects of Dietary Inclusion of Xylooligo- Saccharides, Arabinoxylooligosaccha- Rides and Soluble Arabinoxylan on the Microbial Composition of Caecal Contents of Chickens.” Journal of the Science of Food and Agriculture 88, no. 14 (2008): 2517–22. https://doi.org/10.1002/jsfa.3373.
Ribeiro, T., V. Cardoso, L.M.A. Ferreira, M.M.S. Lordelo, E. Coelho, A.S.P. Moreira, M.R.M. Domingues, M.A. Coimbra, M.R. Bedford, and C M Fontes. “Xylo-Oligosaccharides Display a Prebiotic Activity When Used to Supplement Wheat or Corn-Based Diets for Broilers.” Poultry Science 97, no. 12 (2018): 4330–41. https://doi.org/10.3382/ps/pey336.
Pollet, Annick. “Functional and Structural Analysis of Glycoside Hydrolase Family 8, 10 and 11 Xylanases with Focus on Bacillus Subtilis Xylanase A,” 2010. https://www.biw.kuleuven.be/m2s/clmt/lmcb/publications/docs/apollet


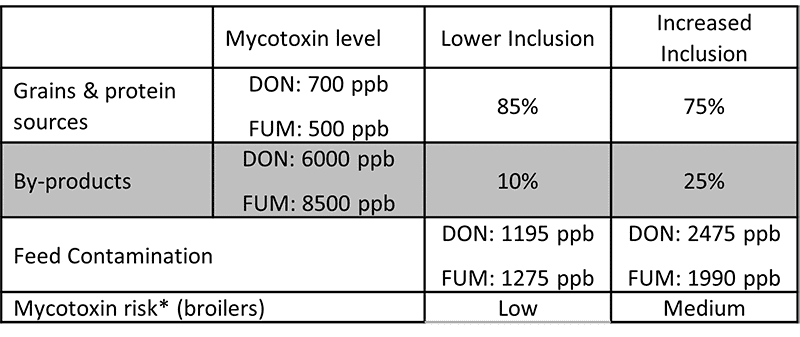
 Destillers’ dried grains with solubles (DDGS), a by-product of ethanol production, is a valuable feed ingredient, particularly as a source of protein for ruminants and monogastric animals at a competitive price.
Destillers’ dried grains with solubles (DDGS), a by-product of ethanol production, is a valuable feed ingredient, particularly as a source of protein for ruminants and monogastric animals at a competitive price.