By Technical Team, EW Nutrition India
Consumer demand drives egg production. With 10 billion people on the planet by the year 2050 (1), producers are under more pressure to provide more protein of higher quality. Modern production practices help extend the laying cycle of commercial flocks to 90–100 weeks. The volume of eggs produced worldwide has thus increased by more than 100% since 1990. Consumers are pushing not just for more eggs, but also for larger eggs. Due to these shifting requirements, farmers and integrators are under pressure to meet demand. As a result, the birds are under metabolic stress to meet needs, which can compromise eggshell quality, laying consistency, and gut health.
Gut health is a key factor in achieving maximum productive potential and laying rate, not only because it’s a key factor for digestion and the absorption of nutrients but also because it’s an essential component of the bird’s immune system.
In today’s layer production, when the cycle is increasing and overall demand to limit the use of antibiotics is growing, laying persistency, eggshell quality, and gut health are critical topics. But what does a laying hen’s healthy gut mean?
Birds need a healthy gut to maximize production. Genetics, nutrition, management and biosecurity all affect production parameters. A gut with a diverse pH and healthy microbiota prevents infections. Gut health is affected by Goblet cells, paneth cells, endocrine cells, absorptive enterocytes, tight junctions, GALT, and mucus. To deal with potential challenges and ensure optimal bird performance, a complex approach is needed, consisting of optimal carbohydrates, proteins, amino acids, minerals, vitamins, enzymes, organic acids, and management strategies.
Vital amino acids, Zn, Vit E, Se, etc. must be supplemented according to the production status and environment to establish good immunity. Maximum production requires a stress-free, hormonally balanced, clean environment, as well as optimal nutrition. Especially given the push for reduced antibiotics and rising welfare and food standards, particularly from the expansion in cage-free farming, producers need to pay considerable attention to the issues of maintaining a healthy gut with these added challenges. Several aspects must be considered when it comes to gut health.
Factors affecting layer gut health
- Breed
- Management
- Environment
- Diet – Nutrients and Anti-Nutritional Factors (ANFs)
- Feed management
- Stress
- Toxins (Mycotoxins and endotoxins)
- Pathogens
- Microbiota
- Parasites
- Physiology
- Metabolism
- Immunity
- Endocrine system
Feed and water are essential
Both vectors create a connection between the external and internal environment of the hen, increasing the possibility of a negative effect on the intestinal balance.
Some common influences:
- Anti-nutritional factors (non starch polysaccharides and anti-trypsic factors)
- Water, raw material and feed contaminants (E. Coli, Salmonella, mycotoxins (Fig. 1) etc.)
- Sudden changes in formulation
- High density diets – excess of nutrients
- Bird physiology. How, different organs and the endocrine system respond against challenges
- Gut microbiota. Represented by the balance between pathogenic and commensal flora. Latter being the one involved in the development of intestinal morphology and structure, immune modulation and supporting digestion and absorption processes.
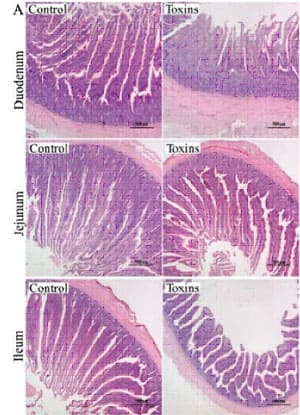 Fig. 1: Effects of dietary mycotoxins on histopathology of the duodenum, jejunum, and ileum Adapted from Zhao et al., 2021 (3)
Fig. 1: Effects of dietary mycotoxins on histopathology of the duodenum, jejunum, and ileum Adapted from Zhao et al., 2021 (3)
Layer gut health: An increasing concern
Nowadays the gut health of the layer matters more than ever. In many countries, consumer preferences have been shifting towards eggs produced in non-cage environments and, in these new housing systems, birds are in closer contact with the litter and are more prone to the proliferation of gut pathogens.
Traditionally, layers were housed in cages, which benefited egg producers by making better use of available space and increasing productivity. This resulted in more birds per house, more automated operations, better management, improved hygiene, decreased incidence of infectious diseases, and cheaper feed consumption and production costs.
Cages, on the other hand, pose other health and welfare concerns. They limit or prevent mobility, ground scratching, wing-flapping, and soaring. As a result, there is increasing pressure for birds to be cage-free and, eventually, free-range. The European Commission stated that, by the end of 2023, a legislative proposal will phase out, and eventually prohibit, the use of cages for a variety of farm animals, resulting in an increase in the number of layers reared in a cage-free system.
According to the Egg Track Report (2021), 219 egg farmers, retailers, food service firms, and hotel chains have pledged to transition completely to cage-free eggs by 2025, with 47 of these companies expanding their commitments to encompass their global supply. This means that farmers and integrators will face increased pressure to migrate from a cage system to a cage-free system. As a result, it is necessary to consider new issues or challenges that may be exacerbated by transitioning to a cage-free production system.
Gut health-associated production losses in laying hens
Water (70%), proteins (10%), and lipids (20%) make up egg yolks. The yolk lipids are triglyceride-rich lipoproteins that are produced in the liver and transferred to the ovary. Cholesterol transported to the egg yolk by lipoproteins is also deposited there, demonstrating the importance of the liver in egg formation.
The gut plays an important role in preventing liver damage by acting as a barrier against dangerous viruses and toxins that could enter the bloodstream and reach this key organ. Efficient feed digestion and absorption of nutrients are essential for the hen to obtain the “material” for maintenance, growth, and egg production.
The Association of Veterinarians of Egg Production in the US found in 2014 that gastrointestinal difficulties cause 40% of health issues during the pullet phase and 50% during production. Coccidiosis, necrotic enteritis, and feed passage were the biggest threats from these gastrointestinal illnesses.
Aging reduces digestive health, causing nutrient digestion and absorption problems and immunological issues. As a result, eggs produced by older hens show increased micro-cracks, gross cracks, and a higher number of dirty eggs.
Eggshell quality issue
Poor intestinal physiology can impair mineral absorption, notably calcium. When this happens, hens utilise the calcium from their bones, but if the problem persists, these stores may diminish and thin-shelled eggs may appear, increasing the percentage of broken eggs. Shell-less eggs are possible.
Bone fractures
In continuation with what was described in the previous point, the bird’s skeletal system weakens due to the use of calcium reserves of the bones, which leads to bone fractures, such as the head of the femur, and other locomotor problems of similar pathogenesis.
Increase in soiled eggs percentage
Deficient intestinal physiology may also cause intestinal flora imbalance. Certain germs proliferate excessively, harming the mucosa and affecting faeces consistency. This raises the number of dirty eggs, which harms consumers due to cross-contamination.
Internal egg quality changes
Due to the alteration of the nutritional function of the intestine, feed digestion and nutrient absorption is affected, and this leads to a decrease in their concentration in the egg. This deficiency causes yolk pigmentation problems, poorer egg nutritional value, and worsening of the Haugh Units, among other issues.
Low egg laying percentage and small egg size
Related to the previous point, the alteration of the nutritional functions of the intestine will also decrease the percentage of egg laying. This is because the bird will not absorb enough nutrients and minerals to cover the needs for egg production (both for the metabolic process and to form the egg). The mentioned problems, derived from inadequate intestinal physiology, lead to poor qualitative and quantitative egg production, which is, in most cases, very difficult to reverse in the short term, and that leads to significant economic losses.
Strategies for gut health maintenance
During the production cycle, the gastrointestinal health of laying hens has a substantial impact on both efficiency and profitability. During peak egg production, chickens often cannot consume enough feed to meet their protein and calcium requirements. This stress can disrupt the gut microbiota, resulting in pathogenic bacterial outbreaks. Infections with Escherichia coli and Clostridium perfringens are prevalent in laying hens. Antibiotics would be administered to the birds to deter severe mortality.
Antibiotics, on the other hand, have hidden costs because eggs produced during antibiotic treatment and withdrawal cannot be marketed for human consumption. Furthermore, antibiotic misuse, such as using too little or for too short a time, might contribute to the development of antibiotic resistance.
A variety of non‐drug substances have been promoted as aids to enhance gut health and to mitigate the risks of coccidiosis and necrotic enteritis in antibiotic‐free production. These products include phytogenic additives, probiotics, prebiotics, organic acids, yolk immunoglobulins, bacteriophages, yeast products, and others.
Probiotics and competitive exclusion (CE) cultures are available for hatchery application, usually by spray, and most of the alternatives are available for feed or water administration. Because of logistical issues, producers usually prefer feed‐administered products, especially if intended for large‐scale applications for prevention.
General water management guidelines
- Ensure adequate cleaning between flocks:
- Removing biofilm (e.g., 25-50 ppm Hydrogen peroxide in the water line for 24-72 hours, then flush)
- Removing scale (target a pH of 5 with weak acid, e.g. citric acid – leave in line for 24 hours, then flush)
- Prior to bird arrival
- Use bleach solution in standing water
- Flush just before birds arrive
- Throughout the life of the flock
- Sanitize (e.g., Chlorine [2-4 ppm] or Chlorine dioxide [0.8 ppm])
- Acidify water (pH 5.5-7)
- Perform waterline biofilm removal at regular intervals throughout the life of the flock (biofilms can form in 6 weeks)
- Routinely check ORP (oxygen reduction potential) at the drinker furthest from the water tank to check the efficacy of sanitation; it should be >650 mv)
Gut health additives
Many gut health solutions can be added to water, included in feed at the feed mill, or top-dressed at the farm. Gut health supplements work differently, making selection challenging. Some gut health products encourage beneficial bacteria, gut tissue formation, digestion, or pathogen inhibition. Thus, while choosing a gut health product, it’s important to determine the root cause of the challenge and make sure the product can address the problem.
The right products are also effective in antibiotic reduction initiatives. However, their prophylactic use should be considered as an alternative option. A strategic method is to deliver a gut-friendly substance at key periods in the chicken’s life.
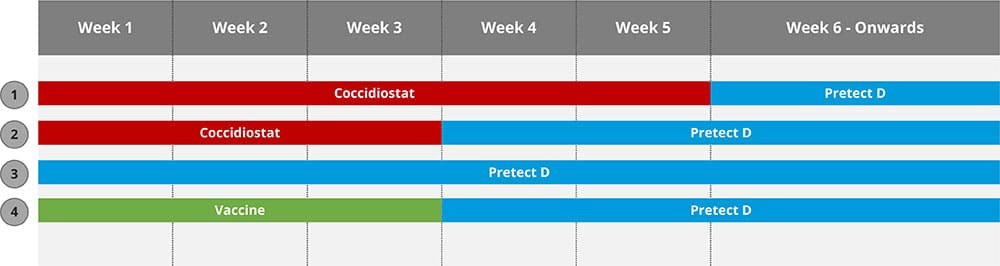
What are the key periods?
Development, transition, and maintenance are three primary stages in the gut development chain (see Figure 2).
- Promote bacterial colonization as well as tissue and immunological improvement during development.
- The transition stage is when feed changes, vaccinations, and handling affect the intestinal environment. These events can alter the gut environment and enhance malabsorption and bacterial overgrowth.
- The gut has ceased developing and reached balance in the maintenance stage, but management or microbial problems can upset it, thus gut tissue support is still necessary.
Understanding the needs of the gut at different points in the bird’s life and the main goals of gut health support at these times is important when designing gut health strategies.
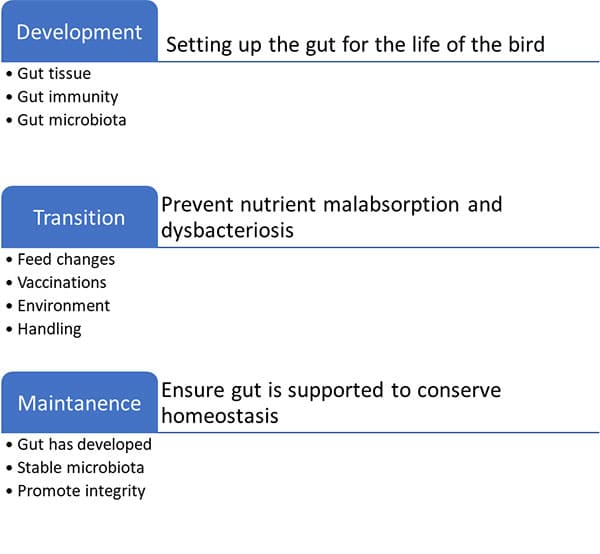 Fig2: Gut need assessment and management strategy
Fig2: Gut need assessment and management strategy
Phytomolecules mitigate gut health challenges
Multiple scientific studies highlight phytomolecules as one of the key elements in antibiotic-free production. These substances support digestion and improve the utilization of nutrients, resulting in a higher daily weight gain, uniform flock, and better feed utilization.
They also have a proven anti-inflammatory effect, as shown in Figure 3. NF-κB is a critical regulator for the expression of genes involved in inflammation. It has been demonstrated that NF-κB plays a novel role in the mechanism of increased epithelial permeability induced by inflammatory factors (including LPS and TNF-α) (5).
Phytomolecules, when combined with effective delivery and synergistic value inside the animal, also have a proven antimicrobial effect and help prevent the development of resistance. Various forms of stresses and insults from feed water and the environment cause oxidative stress and thereby impaired tight junctions, resulting in a leaky gut. Leaky gut has multiple consequences, ranging from poor flock performance to wet litter and raised ammonia levels. Phytomolecules are well documented for their NF-κB inhibitory and anti-inflammatory properties (6). They also help curb oxidative stress and maintain gut integrity. The right product will also be mild on the beneficial flora, showing selective antimicrobial activity and preserving the balance of the gut microbiota.
Finding the right product (perfect formulation and technology to counter high volatility, offer high thermostability, and yet provide effective delivery inside the animal) is of paramount importance for desired results.
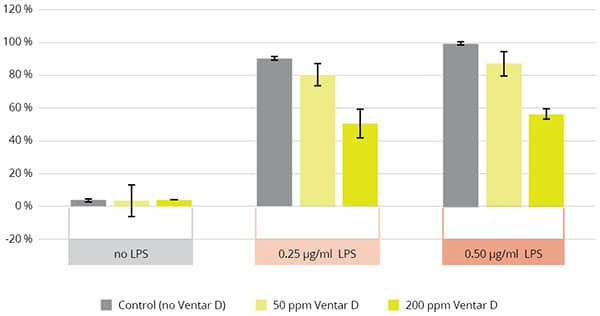 Figure 3. NFkB activity with phytomolecule-based product Ventar D (EW Nutrition)
Figure 3. NFkB activity with phytomolecule-based product Ventar D (EW Nutrition)
Conclusion
Optimal growth and FCR in food-producing animals depend on intestinal health. Researchers have studied gut flora, function, and immunity. Regional variations in chicken production, management styles, environment, disease challenge, and feed raw materials complicate gut health maintenance. Consequently, appropriate bird management techniques are essential to bird health, welfare, and performance.
Due to the recent focus on reducing or restricting antibiotic use, intestinal problems have increased, often resulting in productivity losses. This has led to the development of several feed additives that can improve intestinal microbiota, prevent pathogens from adhering to epithelial cells, and boost immune response.
Probiotics, prebiotics, organic acids, organic acid blends (protected or not), phytobiotics, and feed enzymes are everywhere. Feed additive performance depends on parameters like hen age, management, production method, genetics, etc. It also depends on additive formulation, a multi-layered mode of action, and on a coating technology that leads to effective release of ingredients in the GIT.
References:
- https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100#:~:text=COVID%2D19,World%20population%20projected%20to%20reach%209.8%20billion%20in%202050%2C%20and,Nations%20report%20being%20launched%20today.
- Zaefarian, F., Abdollahi, M. R., & Ravindran, V. (2016). Particle size and feed form in broiler diets: impact on gastrointestinal tract development and gut health. World’s Poultry Science Journal, 72(2), 277-290.
- Zhao L, Feng Y, Wei J-T, Zhu M-X, Zhang L, Zhang J-C, Karrow NA, Han Y-M, Wu Y-Y, Guo Y-M, Sun L-H. Mitigation Effects of Bentonite and Yeast Cell Wall Binders on AFB1, DON, and OTA Induced Changes in Laying Hen Performance, Egg Quality, and Health. Toxins. 2021; 13(2):156. https://doi.org/10.3390/toxins13020156
- He, F., Peng, J., Deng, X. L., Yang, L. F., Camara, A. D., Omran, A., … & Yin, F. (2012). Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine, 59(2), 264-272.
- Dragoș, D., Petran, M., Gradinaru, T. C., & Gilca, M. (2022). Phytochemicals and Inflammation: Is Bitter Better?. Plants, 11(21), 2991.