The hidden danger of endotoxins in animal production
Find out more about endotoxins here
Find out why LPS can cause endotoxemia and how intelligent toxin mitigation solutions can support endotoxin management.
Each E. coli bacterium contains about 100 lipopolysaccharides molecules in its outer membrane
Lipopolysaccharides (LPS) are the major building blocks of the outer walls of Gram-negative bacteria. Throughout its life cycle, a bacterium releases these molecules, which are also known as endotoxins, upon cell death and lysis. The quantity of LPS present in Gram-negative bacteria varies between species and serotypes; Escherichia coli, for example, contain about 100 LPS/bacterial cell. When these are released into the intestinal lumen of chickens or swine, or in the rumen of polygastric animals, they can cause serious damage to the animal’s health and performance by over-stimulating their immune system.
How lipopolysaccharides cause disease
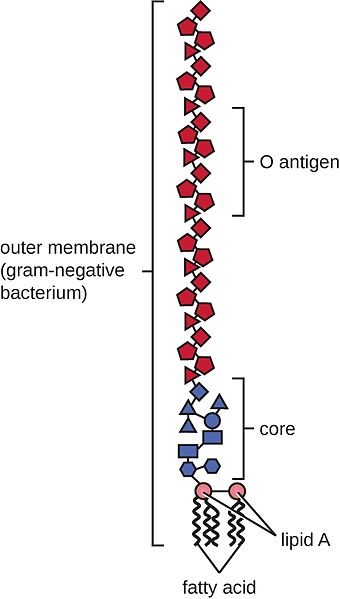
LPS are rather large and structured chemical molecules with a weight of over 100,000 D. They are highly thermostable; boiling in water at 100°C for 30 minutes does not destabilize their structure. LPS consist of three chemically distinct sections: a) the innermost part, lipid A, consisting mostly of fatty acids; b) the core, which contains an oligosaccharide; and c) the outer section, a chain of polysaccharides called O-antigen (Figure 1).
Figure 1: Structure of an LPS
The toxicity of LPS is mainly caused by lipid A; however, both lipid A and O-antigen stimulate the immune system. This happens when the LPS pass the mucosa and enter the bloodstream or when they attack the leukocytes.
The intestinal mucosa is the physical immune barrier that protects the microvilli from external agents (bacteria, free LPS viruses, etc.). Despite its strength (the thickness, for example, amounts to ≈830 µm in the colon and ≈123 µm in the jejunum), vulnerable points exist (cf. Zachary 2017).
LPS can easily come into contact with the cells of the lamina propria (a layer of connective tissue underneath the epithelium) through the microfold (M) cells of the Peyer’s patches (which consist of gut-associated lymphoid tissue). The M cells are not covered by mucus and thus exposed.
Secondly, LPS can also pass through the mucosa, where they become entangled in this gelatinous structure. There, they come into contact with the lymphocytes or can reach the regional lymph nodes through the afferent lymphatic vessels.
Thirdly, LPS might affect the tight junctions, the multiprotein complexes that keep the enterocytes (cells that form the intestinal villi) cohesive. By destabilizing the protein structures and triggering enzymatic reactions that chemically degrade them, LPS can break the tight junctions, reaching the first capillaries and, consequently, the bloodstream.
The presence of endotoxins in the blood, endotoxemia, can trigger problematic immune responses in animals. An innate immune stimulation leads to an increase in the concentration of pro-inflammatory cytokines in the blood and, consequently, to an induced febrile response in the animal: heat production increases, while the available metabolic energy decreases. As a result, performance suffers, and in the worst-case scenario, septic shock sets in. Furthermore, when LPS compromise intestinal integrity, the risk of secondary infections increases, and production performance may decline.
LPS’ modes of action
How does all of this happen? The physiological consequences of endotoxemia are quite complex. Simplified, the immune system response to LPS in the blood takes three forms:
- The stimulation of TLR4 (toll-like receptor 4) induces monocytes and macrophages to secrete critical pro-inflammatory cytokines, primarily interleukin (IL) IL-1β, IL-6, IL-8, and tumor necrotic factor (TNF) α and β. TLR4 is a structure on the cell membrane of mainly macrophages and leukocytes, which is activated by the LPS-binding protein (LBP).
- The complement cascade constitutes about 10% of plasma proteins and determines the chemotaxis and activation of leukocytes. It can form a membrane attack complex (MAC), which perforates the membranes of pathogenic cells, enabling lysis.
- The Hagemann factor, also known as coagulation factor XII: once stimulated by LPS, it initiates the formation of fibrin (through the intrinsic coagulation pathway), which might lead to thrombosis. The Hagemann factor directly stimulates the transformation of prekallikrein to kallikrein (enzymes involved in regulating blood pressure).
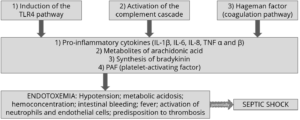
Figure 2: How LPS leads to endotoxemia – 3 modes of action
These three modes of action of inflammatory stimulation lead to important physiological reactions:
- Pro-inflammatory cytokines (see above) modulate the functional expression of other immune cell types during the inflammatory response;
- Metabolites of arachidonic acid (prostaglandins, leukotrienes, and lipoxins), intra- and extracellular messengers that influence the coagulation cascade;
- Synthesis in the blood of bradykinin, a peptide responsible for the typical symptoms of inflammation, such as swelling, redness, heat and pain;
- PAF (platelet-activating factor), which creates inflammatory effects through narrowing of the blood vessels and constriction of the airways, but also through the degranulation of leukocytes.
The symptoms of endotoxemia are: hypotension, metabolic acidosis, hemoconcentration, intestinal hemorrhage, fever, activations of neutrophils and endothelial cells, and predisposition to thrombosis.
In case of a progression to septic shock, the following sequence takes place:
1) Reduction in blood pressure and increased heart rate (hemodynamic alterations)
2) Abnormalities in body temperature
3) Progressive hypoperfusion at the level of the microvascular system
4) Hypoxic damage to susceptible cells
Up to here, symptoms follow a (severe) endotoxemia pathogenesis. A septic shock furthermore entails:
5) Quantitative changes in blood levels of leukocytes and platelets
6) Disseminated intravascular coagulation (see Hageman factor)
7) Multi-organ failure
8) Death of animal
If an animal is continously challenged with endotoxins, experiences septic shock, or comes close to it, it risks developing LPS tolerance, also known as CARS (compensatory anti-inflammatory response syndrome). This syndrome essentially depresses the immune system to control its activity. The anti-inflammatory prerogative of CARS is not to interfere directly with the elimination of pathogens but to regulate the “excessive” inflammatory reaction in a hemostatic way. However, this regulation can be extremely dangerous as the syndrome involves a lack of homeostasis control, and an excessive depression of the immune system leaves the organism exposed to the actual pathogens.
Farm animal research on endotoxemia pathogenesis
Lipopolysaccharides are difficult to quantify in the intestine of a live animal. One way to evaluate a possible endotoxemia is to analyze biomarkers present in the bloodstream. The most important one is the LPS themselves, which can be detected in a blood sample taken from the animal via ELISA. Other biomarkers include pro-inflammatory interleukins, such as TNF α and β, IL-6 or IL-8, and fibrin and fibrinogen (though they are not specific to endotoxemia). It is vital to carry out a blood sample analysis to deduce a possible endotoxemia from symptoms and performance losses in the animal.
How the metabolic effects of endotoxemia depress performance
One of the biggest issues caused by endotoxemia is that animals reduce their feed intake and show a poor feed conversion rate (FCR). Why does this happen? The productive performance of farm animals (producing milk, eggs, or meat) requires energy. An animal also requires a certain baseline amount of energy for maintenance, that is, for all activities related to its survival. As a result of inflammation and all those physiological reactions mentioned above, endotoxemia leads to a feverish state. Maintenance needs to continue; hence, the energy required for producing heat will be diverted from the energy usually spent on producing milk, egg, meat, etc., and performance suffers.
The inflammation response can result in mitochondrial injury to the intestinal cells, which alter the cellular energy metabolism. This is reflected in changes to the levels in adenosine triphosphate (ATP), the energy “currency” of living cells. A study by Li et al. (2015) observed a respective reduction of 15% and 55% in the ATP levels of the jejunum and ileum of LPS-challenged broilers, compared to the unchallenged control group. This illustrates the extent to which animals lose energy while they experience (more or less severe) endotoxemia.
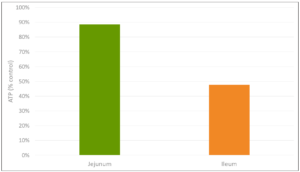
Figure 3: Reduction in ATP level in Jejunum and Ileum in broilers (adapted from Li et al., 2015)
A piglet study by Huntley, Nyachoti, and Patience (2017) took this idea further (Figure 4): 3 groups of 10 Yorkshire x Landrace pigs, weighing between 11 and 25 kg, were studied in metabolic cages and in respiratory chambers. This methodology allows for simultaneous measurement of oxygen consumption, CO2 production, energy expenditure, physical activity, and feed/water intake. The study found that LPS-challenged pigs retained 15% less of the available metabolizable energy and showed 25% less nutrient deposition. These results show concrete metabolic consequences caused by the febrile response to endotoxemia we discussed above.
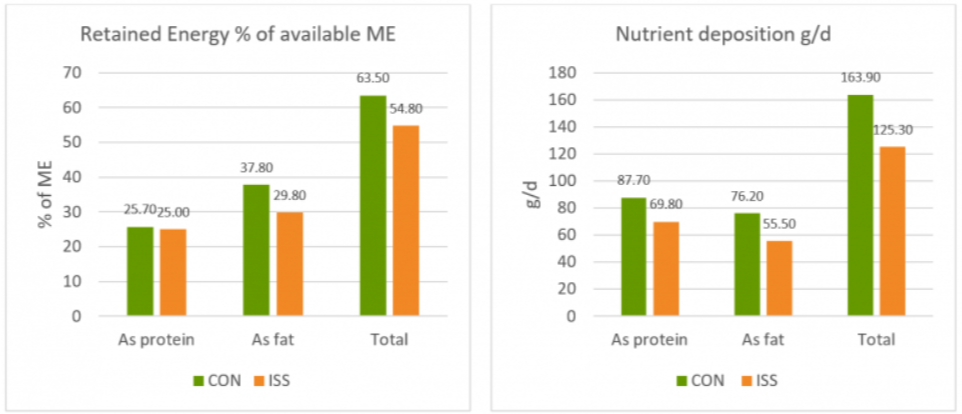
Figure 4: Retained Energy as % of ME intake and nutrient deposition of pigs in metabolic cages (adapted from Huntley, Nyachoti, and Patience, 2017)
Control treatment (CON) = Pigs fed by a basal diet
Immune system stimulation treatment (ISS) = Pigs given LPS (E. coli serotype 055:B5) injection
A loss of energy retained due to a reduction in available metabolizable energy leads to losses in performance as the amount of energy available for muscle production and fat storage will be lower. Furthermore, the decrease in feed intake creates a further energy deficit concerning production needs.
A trial carried out at the University of Illinois examined the effects of repeated injections of 400 μg E. coli LPS on chick performance from 11 to 22 days after hatching. The chicks were fed casein-based diets with graded levels of arginine. LPS administration reduced weight gain (P<0.05) and feed intake, and these effects tended to be worse at higher levels of arginine supplementation (Figure 5). The researchers hypothesize that, in response to endotoxin and elevated cytokine levels, macrophages use more arginine to produce nitric oxide, diverting it from protein production for muscle development.
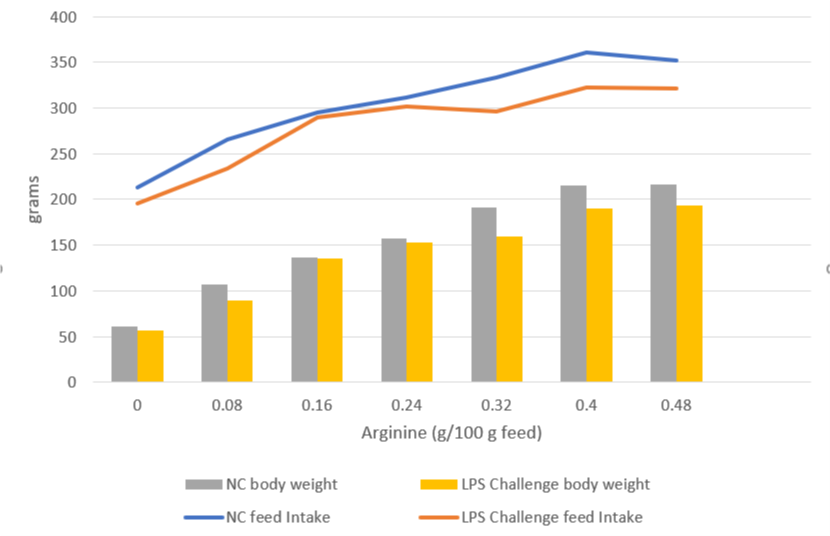
Figure 5: Effects of LPS on feed intake and body weight gain in chicks fed graded level of arginine (based on Webel, Johnson, and Baker, 1998)
NC = negative control
This data on poultry complements the results for swine, again showing that endotoxin-induced energy losses quantifiably depress animal performance even in milder disease cases.
The way forward: Endotoxin mitigation
Animals suffering from endotoxemia are subject to severe metabolic dysfunctions. If they do not perish from septic shock, they are still likely to show performance losses. Moreover, they at great risk of immunosuppression caused by the immune system “overdrive.” Effective endotoxin mitigating agents can help to prevent these scenarios.
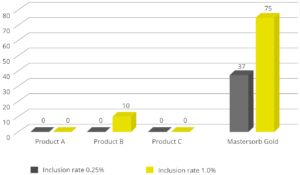
EW Nutrition’s Mastersecure Gold is not only a leading anti-mycotoxin agent; thanks to its specific components, it effectively binds bacterial toxins. An in vitro study conducted at the Hogeschool Utrecht laboratory (part of Utrecht University) evaluated the binding capacity of Mastersecure Gold on LPS compared to three different competitor products. All products were tested at two different inclusion rates. At an inclusion rate of 0.25%, only Mastersecure Gold reduced the toxin load on the solution by 37%. At 1% inclusion, Mastersecure Gold (noted as Mastersorb below) bound 75% of the toxin, while only one competitor product demonstrated any binding (10%).
Lipopolysaccharides are a constant challenge for animal production. The quantity of Gram-negative bacteria in an animal intestine is considerable; therefore, the danger of immune system over-stimulation through endotoxins cannot be taken lightly. Producers need to prioritize the maintenance of intestinal eubiosis in production animals proactively; for instance, through targeted gut health-enhancing additives based on phytomolecules and, possibly, organic acids.
Most importantly, the detrimental impact of LPS can be mitigated by using a high-performance agent such as Mastersecure Gold. To limit losses from an energy point of view yields positive results in terms of production levels and the prevention of secondary infections, preserving animal health and farms’ economic viability.
By Claudio Campanelli, EW Nutrition
References
Adib-Conquy, Minou, and Jean-Marc Cavaillon. “Compensatory Anti-Inflammatory Response Syndrome.” Thrombosis and Haemostasis 101, no. 01 (2009): 36–47. https://doi.org/10.1160/th08-07-0421.
Huntley, Nichole F., C. Martin Nyachoti, and John F. Patience. “Immune System Stimulation Increases Nursery Pig Maintenance Energy Requirements.” Iowa State University Animal Industry Report 14, no. 1 (2017). https://doi.org/10.31274/ans_air-180814-344.
Li, Jiaolong, Yongqing Hou, Dan Yi, Jun Zhang, Lei Wang, Hongyi Qiu, Binying Ding, and Joshua Gong. “Effects of Tributyrin on Intestinal Energy Status, Antioxidative Capacity and Immune Response to Lipopolysaccharide Challenge in Broilers.” Asian-Australasian Journal of Animal Sciences 28, no. 12 (2015): 1784–93. https://doi.org/10.5713/ajas.15.0286.
Mani, Venkatesh, James H Hollis, and Nicholas K Gabler. “Dietary Oil Composition Differentially Modulates Intestinal Endotoxin Transport and Postprandial Endotoxemia.” Nutrition & Metabolism 10, no. 1 (2013): 6. https://doi.org/10.1186/1743-7075-10-6.
Webel, D.M., R.W. Johnson, and D.H. Baker. “Lipopolysaccharide-Induced Reductions in Body Weight Gain and Feed Intake Do Not Reduce the Efficiency of Arginine Utilization for Whole-Body Protein Accretion in the Chick.” Poultry Science 77, no. 12 (1998): 1893–98. https://doi.org/10.1093/ps/77.12.1893.
Zachary, James F. “Chapter 4 – Mechanisms of Microbial Infections.” Essay. In Pathologic Basis of Veterinary Disease, 132–241. St Louis, MO: Mosby, 2017. https://doi.org/10.1016/B978-0-323-35775-3.00004-7.