Understanding the dangers of mycotoxins for breeder hens

As the producers of hatching eggs and day-old chicks, breeding hens are the backbone of the poultry industry. Hence it is common practice to pay particular attention to this valuable asset’s feed, selecting raw materials of high nutritional quality and safety. However, in any feed formulated for animals in production and reproduction, studies show that it is almost inevitable to find a certain level of mycotoxin contamination.
Mycotoxins exert toxic effects mainly on the gastrointestinal tract, liver, and kidneys and can accumulate in some tissues but also in the eggs. Mycotoxin contamination in breeder hens rations does not always lead to visible symptoms, such as when trichothecenes cause oral lesions. However, it may influence productivity, egg quality, hatchery performance, as well as chick quality and immunity. Mycotoxin risk management is thus an essential part of managing breeder hens. Mycotoxins can negatively affect eggshell quality and, as a consequence, embryonic mortality.

By Technical Team, EW Nutrition.
Type of mycotoxin and exposure time determine effect on egg production
Mycotoxicosis in hens can cause reduced egg production, most likely because it causes a decrease in protein synthesis. A lower synthesis of albumin results from a degeneration of the liver tissue due to aflatoxin, ochratoxin, T2 and DON exposure. The liver then may look pale, friable and occasionally shows superficial hemorrhages.
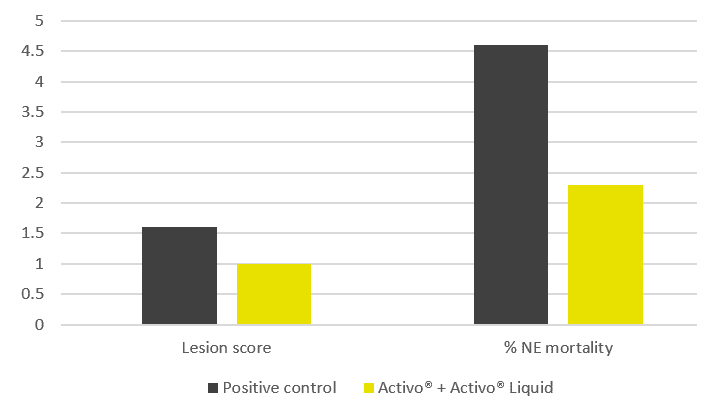
The contamination levels at which these effects can be observed are as low as 100ppb in feed, for example, during a 21-day exposure to ochratoxin (Figure 1). With increasing levels of the toxin, production further decreases. A similar effect is observed when breeder hens are exposed to aflatoxins.

Figure 1 – Effect of mycotoxins on egg production, compared to non-contaminated control (=100 %)
Egg production, however, is not the only parameter that is affected when breeding hens are exposed to mycotoxins. Earlier on in the reproductive cycle, they already impact on embryonic mortality and hatchability. These effects are potentially more severe and may even occur without any noticeable change in the number of eggs produced.
Mycotoxins’ insidious consequences for eggshell quality and embryonic mortality
The eggshell is important to protect the progeny: thin and fragile shells can increase embryonic mortality, lower embryonic weight gain and decrease hatchability. Eggshell quality is a function of the hen’s calcium and vitamin D3 metabolism. The bioavailability of calcium and of vitamin D3 depends on intestinal integrity and on the production of enzymes and transporters that aid in feed metabolism. These processes can be adversely affected by aflatoxins, DON, T2, and Fumonisins.
The gastrointestinal tract is not the only site of mycotoxin action, however. Mycotoxins such as aflatoxins and ochratoxins have nephrotoxic effects, affecting calcium metabolism and increasing its excretion via the urine, while lowering its levels in blood serum.
Moreover, mycotoxins damage the liver, which plays a central role in egg production, being responsible for vitamin D3 metabolism and the synthesis of the lipids that make up the yolk. Moreover, the synthesis of transporters for lipids, calcium, and carotenoids ̶ important components of the egg ̶ also takes place in the liver. When liver function is impaired, the internal and external quality of the egg declines, which, in the end, affects the production of day-old chicks.
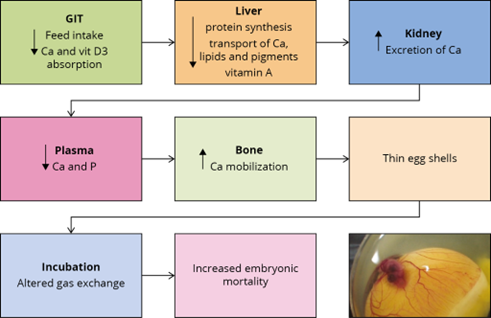
Figure 2 – Effects of mycotoxins on eggshell quality and embryonic mortality
Figure 2 summarises the possible ways in which mycotoxins can negatively affect eggshell quality and, as a consequence, increase embryonic mortality. If a hen’s intestinal integrity is compromised, the utilization of nutrients decreases. Liver and kidney damage leads to a diminished availability of calcium and other nutrients necessary for egg formation. The birds’ calcium (and phosphorus) levels in the plasma are then lower and may lead to a greater mobilization of calcium from the bones. However, this response cannot be maintained and the eggs get a thinner shell.
The thickness of the eggshell influences the egg’s moisture loss and exchange with the environment during the incubation period. An eggshell of optimal quality does not allow the loss of nutrients and prevents bacterial contamination. Thinner eggshells are less able to fulfill these functions, leading to higher embryo mortality.

Figure 3 – Effects of mycotoxins on embryonic mortality
Figure 3 shows the effect of different mycotoxins on embryonic mortality. Incremental levels of ochratoxin or aflatoxin heighten embryonic mortality in a range from 1.5 to 7.5 times the embryonic mortality of the control group. In some cases, embryos are affected even when the hens received feed contaminated with mycotoxin levels that are within the guidelines suggested by the EFSA.
For example, an exposure to 4900ppb of DON for ten weeks increases the number of embryos with abnormalities. The causes are not entirely clear, as only traces of DON can be found in the egg. However, we do know that this mycotoxin can affect the protein synthesis at the level of the hen’s liver and therefore compromise the deposition of nutrients into the egg.
Mycotoxins’ effects on the progeny may cause long-term damage
Ochratoxin and aflatoxin can be transferred into the egg, where they exert toxicity on the embryos. This does not necessarily result in mortality. However, the chicks can suffer from a compromised immune function due to two reasons: lower transmission of antibodies from the hen and lower viability of the chickens’ immune cells, accompanied by a lower relative weight of the bursa of Fabricio and the thymus.
When both aflatoxin and ochratoxin are present in the feed, the effect on these parameters is synergistic. As a consequence of mycotoxin contamination, the animals’ immune response is impaired, which makes them more susceptible to infection. The final result could be increased early chick mortality due to a higher incidence of bacterial and viral infections.
The transmission of other mycotoxins into the egg is minimal. While this means that a direct effect on the progeny is unlikely to occur, mycotoxin contamination still has a snowball effect: we have to consider the indirect effect of a lower deposition of nutrients on chick quality.
Prevention is key: mycotoxin risk management for breeder hens
The best approach to manage mycotoxin risk is to implement an integrated strategy that includes good crop and grain storing practices, regular raw material sampling and mycotoxin evaluation and analysis. Management tools (such as MasterRisk) can help to evaluate mycotoxin interactions and to choose the best strategy for dealing with specific mycotoxin challenges.
The results of mycotoxin analyses can be used to take decisions regarding the inclusion levels of raw materials and in choosing feed additives that counteract mycotoxins. Products based on plant extracts, yeast cell walls, and clay minerals can help to stabilize a digestive system challenged by mycotoxins. They support the barrier function in the intestine, preventing the passage of mycotoxins into the bloodstream.
Phytomolecules are another piece of the puzzle: thanks to their antimicrobial, anti-inflammatory and antioxidant properties, they support liver function. This is particularly important for long-living animals prone to accumulating mycotoxins in their body tissues.
For a long time the “deleterious effects” of mycotoxins on breeder hens and “their repercussions on progeny health status and performance have not received from a scientific point of view as much attention”(Calini and Sirri, 2007) as they ought to have. However, now that the dangers of mycotoxins for breeder hens’ welfare, health and performance are better understood, it is clear that mycotoxin risk evaluation and management is central to successful poultry production.
*This article first appeared in All About Feed on 31 October 2018
Reference:
Photo: Hans Prinsen.