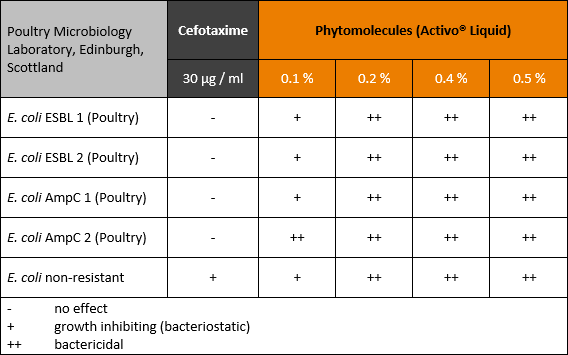
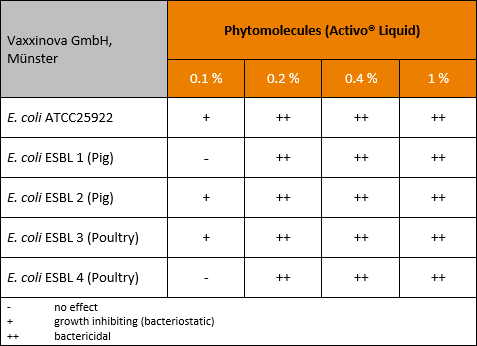
Feeding layers for longer laying cycles and optimized production

Conference report
At the recent EW Nutrition Poultry Academy in Jakarta Indonesia, Dr Steve Leeson, Professor Emeritus, University of Guelph, Canada, commented that “genetic progress in layer breeding has been substantial in recent decades. Since 1995, the yearly change has included +1 egg, -0.01 feed/dozen eggs, -10g final bodyweight, 0.02% mortality, and +1 week at >90% egg production. This improved persistency of commercial laying hens enables egg producers to keep flocks longer in production, provided egg shell quality can be maintained.”
He noted that “the increase in hen-housed egg production is mainly due to longer clutch length and improved uniformity of layer flocks. No doubt, there is a trend in cage layers to longer production cycles. A popular commercial goal is 500 eggs in one cycle with no moult, although this has already been surpassed in many flocks. The modern layer is capable of laying 150 eggs per clutch.”
Dr Leeson, however, stressed that “genetic progress and longer laying cycles have consequences. Long laying cycle programmes start during pullet rearing – you can’t make decisions at 72 weeks of age. Instead, you must start with your end goals, such as persistency, egg size and shell quality, in mind. You can then develop a life-cycle approach to feeding, lighting, nutrition, and general management.” Important issues to manage include:
Body weight control – early and late
Mature body weight dictates subsequent egg size. In the past, the common goal was being at, or above, management guide weight recommendations. For extended lay, a larger body weight results in too large an egg past 70 weeks of age, and so it is more difficult to maintain egg shell quality. Now the goal is to grow a slightly smaller pullet, and emphasis changes to achieving adequate early egg size from this smaller bird. This makes pre-lay nutrition for these slightly smaller pullets even more important.
The scheduling of rearing diets is more important than diet formulation. Dr Leeson’s guidelines are:
- Starter diet – 19-20% CP, 2,850-2,900 kcal ME/kg from day old to target pullet body weight
- Grower diet – 17-18% CP, 2,800-2,900 kcal ME/kg from target body weight to mature body size
- Pre-lay diet (or layer diet?) – 16-18% CP, 2,800-2,900 ME/kg, mature body size to first egg
All nutrients are important, but energy is usually limiting for egg number, whereas protein/amino acids influence egg size (and feathering).
There is now even more emphasis on pullet growing to ensure adequate fat reserves through peak production, so birds are in a positive energy balance. The establishment of an energy reserve occurs during the rearing phase and has a significant effect on the bird’s body composition at point of lay.
Egg size control – early and late
The obvious solution to manage body weight (and egg size) is to light-stimulate a smaller pullet, or at least to not light-stimulate a heavy pullet. This achieves a balance between accepting reduced early egg size, versus limiting an increase in egg size late in the production cycle.
Egg size can be increased in smaller early-lay pullets by:
- Reducing environmental temperature, if possible, to stimulate feed intake
- Midnight feeding 19-29 weeks
- Adequate amino acid nutrition intake, tailored to feed intake, especially methionine
- Increased number of feedings/day and increased feed particle size (pellets)
Shell strength is negatively correlated with egg size. To temper egg size late in the cycle, Dr Leeson recommended:
- Body weight control
- Controlled day length: longer day length = increased feed intake, 14 hours maximum day length in controlled-environment houses
- Warmer temperature – 26oC is ideal
- Reduce number of feedings and particle size
- Temper amino acid nutrition (with caution). Low crude protein/high amino acid diets limit the increase in egg size.
Midnight feeding provides about 1-hour extra light per day and therefore stimulating feed consumption in the middle of the dark period. Having access to feed during this period improves eggshell quality via the supply of calcium during the time when shell calcification takes place. The extra light period is perceived by the bird to be part of the night. The dark period after the light period must be longer than the initial dark period, as the bird perceives the start of the day is the end of the longest period of darkness. Removing midnight feeding should be done gradually – 15 minutes per week, advised Dr Leeson.
Preventing calcium depletion
Also known as cage layer fatigue, calcium depletion is becoming more common in all strains due to high sustained egg output. Calcium deficiency in the feed leads to loss of medullary or long bone (a reservoir of about 4g of calcium) and increased bone fragility. It is commonly seen at 35-40 weeks of age, with a 1-2% occurrence. If the incidence is more than 2%, seek advice for your pre-lay nutrition.
The development of the medullary bones takes about 10 days and requires additional calcium. Pre-lay rations support a smooth transition from developer feed to layer feed, with 2-2.5% calcium, while the other nutrients are similar to a layer feed. Pre-lay rations help the birds to adapt to the high calcium content of layer feed and to maintain sufficient daily feed intake.
To prevent calcium depletion, Dr Leeson suggested:
- Optimise pre-lay calcium (Ca) and phosphorous (P) nutrition
- Intake of 1.5g Ca, 350-450mg available P/day for at least 7 days prior to first egg
- During early lay, ensure 3.5-4 g Ca and 420 mg available P/day
- Consider vitamin D3 water treatment (150 IU/day, twice weekly)
Pre-lay diets provide the bird with the opportunity to deposit medullary bone. This bone deposition coincides with follicular maturation and is under the control of both estrogens and androgens. The latter hormone seems essential for medullary bone growth, and its presence is manifested in the growth and reddening of the comb and wattles. Consequently, there will be little medullary deposition, regardless of diet calcium level, if the birds are not showing comb and wattle development and this stage of maturity should be the cue for increasing the bird’s calcium intake.
Liver health
Excess energy relative to needs results in excess fat accumulation that is prone to oxidation. This is why you never see fatty liver haemorrhagic syndrome (FLHS) in poor-producing flocks. Layers normally have a very fatty liver, as 100% of egg yolk synthesis occurs in the liver.
The lower the fat content of the diet, the greater the stress/need to fat synthesis in the liver. With a low energy/low fat/carbohydrate diet FLHS is almost universal to varying degrees. One treatment is to add fat to the diet! Haemorrhage (not always FLHS) is inevitable with dietary omega-3s that are very prone to oxidation.
Dr Leeson recommended prevention/control for FLHS, which usually starts about weeks 36-40, including:
- +1.0 kg choline
- +0.5 kg methionine
- +100 IU vitamin E
- +30% does Hy-D because of impaired liver metabolism of vitamin D3 (that can also impact calcium absorption)
- Add 2% dietary fat without change in diet energy level
***
EW Nutrition’s Poultry Academy took place in Jakarta and Manila in early September 2023. Dr. Steve Leeson, an expert in Poultry Nutrition & Production with nearly 50 years’ experience in the industry, was the distinguished keynote speaker.
Dr. Leeson had his Ph.D. in Poultry Nutrition in 1974 from the University of Nottingham. Over a span of 38 years, he was a Professor in the Department of Animal &Poultry Science at the University of Guelph, Canada. Since 2014, he has been Professor Emeritus at the same University. As an eminent author, he has more than 400 papers in refereed journals and 6 books on various aspects of Poultry Nutrition & Management. He also won the American Feed Manufacturer’s Association Nutrition Research Award (1981), the Canadian Society of Animal Science Fellowship Award (2001), and Novus Lifetime Achievement Award in Poultry Nutrition (2011).