Cryptosporidia in calves – chickens can help

By Lea Poppe, Regional Technical Manager, EW Nutrition
Diarrhea due to infestation with cryptosporidia is one of the most pressing problems in calf rearing. These protozoa, along with rotaviruses, are now considered the most common pathogens in infectious calf diarrhea. Due to their high resistance and thus limited possible control and prevention measures, they have now overtaken other pathogens such as coronaviruses, salmonellae, and E. coli.
Cryptosporidia show complex development
Cryptosporidia are single-celled intestinal parasites. In calves, Cryptosporidium parvum and Cryptosporidium bovis are most commonly found. C. bovis is normally considered nonpathogenic. Accordingly, the disease known as cryptosporidiosis is caused by C. parvum. The rapid tests for determining the diarrheal pathogens, which are increasingly widespread, are usually unsuitable for distinguishing between the individual strains, which can lead to false positive results.
Resistant in the environment, active in the animal
In the environment, cryptosporidia are distributed as oocysts. The oocysts are only about 5 µm in size and have a very resistant shell. They can remain infectious for up to 6 months in high humidity and moderate temperatures. Drought and extreme temperatures (below -18°C and above 65°C) cause the oocysts to die.
After oral ingestion, the oocysts are reactivated by conditions in the gastrointestinal tract (low pH and body temperature): As sporozoites, the parasites attach to the posterior small intestine, causing diarrhea symptomatology. There, they surround themselves with a special protective membrane, and the complex life cycle continues. Only a few days after infection, reproductive forms are detectable in the calf’s intestine, and excretion of infectious oocysts in the feces begins.
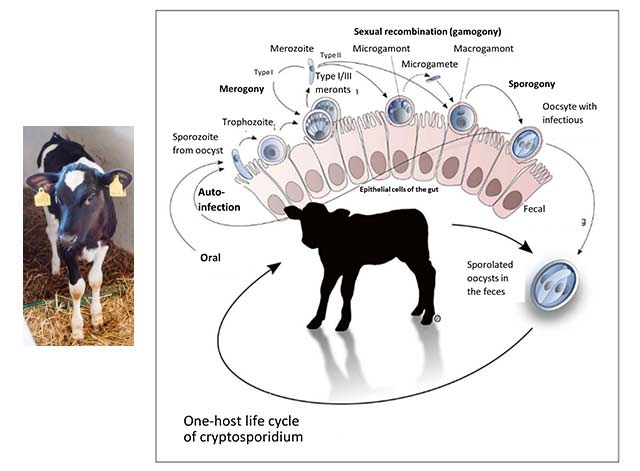
Figure 1 (Olias et al., 2018): Life cycle of cryptosporidia: ingested oocysts release four sporozoites that invade host enterocytes (intestinal epithelial cells). There, they develop into trophozoites before asexual and sexual reproduction ensues, and thin- and thick-walled oocysts are formed. Thick-walled oocysts are excreted through the intestine. Thin-walled oocysts may break apart, and the sporozoites may infect other enterocytes, resulting in relapse or prolonged diarrhea. Infestation of the cells leads to their destruction, resulting in villi atrophy or fusion.
Oocysts bring the disease to the animal
Cryptosporidiosis is transmitted either by direct contact of calves with feces from infected animals or indirectly by ingesting contaminated feed, bedding, or water. Each gram of feces excreted by calves showing symptoms may contain up to 100 million oocysts. According to experimental studies, as few as 17 orally ingested oocysts are sufficient to trigger infection. In addition, some multiplication forms can infect other intestinal cells directly within the intestine and thus further advance the disease by autoinfection.
Cryptosporidiosis caused by cryptosporidia often presents with typical diarrhea symptoms and occurs primarily in calves up to 3 weeks of age. Older calves may also be infected with cryptosporidia but usually show no symptoms. Pathogen excretion and, thus, the spread of disease within the herd is nevertheless likely due to the minimal infectious dose.
Damage to the intestinal wall leads to retardation of growth
Attachment of cryptosporidia to the intestinal wall is associated with an inflammatory reaction, regression and fusion of the intestinal villi, and damage to the microvilli. As a result, nutrient absorption in the small intestine is impaired, and more undigested nutrients enter the colon. The microflora starts a fermentation process with lactose and starch, leading to increased lactate levels in the blood and, thus, hyperacidity in the calf. Faintness, unwillingness to drink, recumbency, and growth disorders are the consequences.
Diarrhea often occurs late or not at all and, accordingly, is not considered the main symptom of cryptosporidiosis. When diarrhea occurs, it lasts about 1-2 weeks. The feces are typically watery, greenish-yellow, and are often described as foul-smelling. Due to diarrhea, there is a loss of electrolytes and dehydration.
Studies show: Cryptosporidia are the most prevalent diarrheal pathogens
Several studies in different regions, which examined calf diarrhea and its triggers in more detail, came to a similar conclusion: Cryptosporidia are one of the most common causes of calf diarrhea. In addition, mixed infections often occur.
| Country or region | Number | Age/Health status | % Crypto-sporidia | % Rota viruses | Combined infections with crypto-sporidia | Others (%) | Source |
| Switzerland | 2 – 21 DL
Ill and healthy |
43 | 46 | 1 case of E. coli | Luginbühl et al., 2012 | ||
| Switzerland | 63 | 1 – 4 DL
Ill and healthy ————– 7 – 20 DL ————– 26 – 49 DL |
34.4
————— 54.0 ————— 33.3 |
3.1
—————- 28.6 —————- 13.3 |
2 EP – 1.6
4 EP – 3.2 —————- 2 EP – 19 3 EP – 3.2 4 EP – 0 —————- 2 EP – 30 3 EP – 11.7 4 EP – 6.7 |
Corona 4.7
E. coli 4.7 Giardia 1.6 ————— Corona 0 E. coli 3.2 Giardia 6.3 ————— Corona 0 E. coli 15 Giardia 35
|
Weber et al., 2016
|
| Switzerland | 147 | Up to 3rd WL;
Diarrhea |
55 | 58.7 | 5.5 % Rota
7.8 % BCV |
Lanz Uhde et al., 2014 | |
| Sweden | 782 | 1 – 7 DL
Diarrhea |
25.3 | Detected with Giardia, E. coli, Rota, Eimeria | Silverlås et al., 2012 | ||
| USA (East coast) | 503 | Pre-weaning | 50.3 | Santin et al., 2004 | |||
| USA | 30 | 2 weeks old
1-8 weeks old 3-12 months 12-24 months |
96.7
45.8 18.5 2.2 |
Santin et al., 2008 | |||
| Germany | 521 | 32 | 9 | Losand et al., 2021 | |||
| Ethiopia | 360 | 18.6 | Ayele et al., 2018 | ||||
| Argentina | 1073 | n.m. / Ill and healthy | 25.5 | Lombardelli et al., 2019 | |||
| UK | n.m. | Ill ?? | 37 | 25 | 20 | Coccidia 8
E. coli 4 Corona 3 Co infections not including Crypto-sporidia 3 |
APHA, SRUC, Veterinary investigation diagnosis analysis (VIDA) report (2014) |
DL = days of life WL = weeks of life n.m. = not mentioned EP = enteropathogen
Cryptosporidia reduces profit
Infection with cryptosporidia and sometimes subsequent diarrhea entails treatment of the animals and generates costs (veterinarian, medication, electrolyte drinks). In addition, poorer feed conversion, lower growth, and animal losses result in lower production efficiency.
A Scottish study shows 34 kg less gain in the first six months of life compared to healthy calves in beef calves that experienced severe cryptosporidiosis in the first three weeks of life. Similar results are described in lambs, also a susceptible species to cryptosporidia. These studies suggest a long-term negative effect of cryptosporidia on growth performance and production efficiency.
Here’s how you can support your calves against cryptosporidia
High resistance of the pathogens to environmental influences, a very low necessary infection dose combined with an elevated excretion of infectious oocysts, and the possibility of autoinfection make cryptosporidia tough opponents. This is also reflected in their worldwide distribution.
What is the treatment?
Suitable drugs for the treatment of cryptosporidiosis are currently unavailable on the market. The only medicine that can be used in case of cryptosporidiosis infestation may only be administered to calves that have had diarrhea symptoms for 24 hours or less. Accordingly, this agent is usually used only for prevention. Scientific studies on its effectiveness are contradictory; some suggest that it merely delays the onset of the disease. In addition, it is not always easy to use due to the exact dosage that must be followed. Doubling the dose (sometimes happening already due to incorrectly observed intervals between doses) can lead to a toxic overdose.
Accordingly, only the symptoms of the disease – diarrhea with its accompanying symptoms – can be treated. Electrolyte and water losses must be continuously compensated with the help of a high-quality electrolyte drink. The buffer substances contained also reduce the hyperacidity of the blood caused by faulty fermentation in the intestines. For successful treatment, the electrolyte drink should be given in addition to the milk drink. Under no circumstances should the feeding of milk or milk replacer be discontinued because the sick calf urgently needs energy and nutrients. Opinions to the contrary are outdated.
As always: prevention is better than treatment
To make it more difficult for cryptosporidiosis to spread from the outset, it is worth looking at the risk factors. These include direct contact with other calves and general herd size. Furthermore, organic farms seem to have more problems with cryptosporidia. Weather also influences calves born during warmer and, at the same time, wetter weather periods (temperature-humidity index) often get sick.
Due to the limited possibilities for treatment, prevention is of greater importance. For other diarrheal pathogens such as rotavirus, coronavirus, and E. coli, it has become established practice to vaccinate dams to achieve better passive immunization of the calf. However, commercial vaccination against cryptosporidia is not currently available, making dam vaccination as unavailable as calf vaccination.
Accordingly, optimal colostrum management is the first way to protect the calf from cryptosporidia infection. This also confirms the general discussion on the Failure of Passive Transfer: various studies suggest that calves with poor immunoglobulin supply suffer from diarrhea more frequently than calves with good supply, although a concrete link to cryptosporidia itself cannot always be established with certainty.
Furthermore, it is essential to break the chain of infection within farms. In addition to the separate housing of the calves, it is necessary to ensure consistent hygiene. One should take advantage of the pathogen’s weakness as well as its sensitivity to high temperatures and ensure that the water temperature is sufficiently high when cleaning the calf pens and calving area. When disinfecting afterward, it is crucial to consider the spectrum of activity of the agent used, as not all are effective against cryptosporidia.
Egg immunoglobulins support animals against cryptosporidia
Egg immunoglobulins were initially designed to help chicks get started. In this process, hens form antibodies against pathogens they are confronted with. As studies have shown, this also works with cryptosporidia. Cama and Sterling (1991) tested their produced antibodies in the neonatal mouse model and achieved a significant (P≤0.001) reduction in parasites there. Kobayashi et al. (2004) registered decreased binding of sporozoites to the intestinal cell model and their decreased viability in addition to oocyst reduction.
In the IRIG Research Institute (2009, unpublished), feeding egg powder with immunoglobulins against cryptosporidia (10 g/day) to 15 calves reduced oocyst excretion. Before administration, calves excreted an average of 106.42 oocysts/g of feces. After administration of egg powder, only two calves still showed 103.21 oocysts/g feces, and the other 13 of the 15 calves showed no oocyst excretion. All these results are confirmed by positive customer feedback on IgY-based feed supplements.
Egg immunoglobulins and optimal colostrum management as a key solution
Since there are no effective drugs against cryptosporidia, animals must be prophylactically protected against this disease as much as possible. In addition to optimal colostrum management, which means feeding high-quality colostrum (IgG≥50g/L) to the calf as soon as possible after birth, we have products with egg immunoglobulins available to support the calf as a prophylactic against cryptosporidia infestation and thus prevent significant performance losses, especially during rearing.
References
Brainard, J., Hooper, L., McFariane, S., Hammer, C. C., Hunter, P. R., & Tyler, K. (2020). Systemic review of modifiable risk factors shows little evidential support for most current practices in Cryptosporidium management in bovine calves. Parasitology research 119, 3572-3584.
Cama, V. A., and C. R. Sterling. “Hyperimmune Hens as a Novel Source of Anti-Cryptosporidium Antibodies Suitable for Passive Immune Transfer.” University of Arizona. Wiley-Blackwell, January 1, 1991. https://experts.arizona.edu/en/publications/hyperimmune-hens-as-a-novel-source-of-anti-cryptosporidium-antibo.
Kobayashi, C, H Yokoyama, S Nguyen, Y Kodama, T Kimata, and M Izeki. “Effect of Egg Yolk Antibody on Experimental Infection in Mice.” Vaccine 23, no. 2 (2004): 232–35. https://doi.org/10.1016/j.vaccine.2004.05.034.
Lamp, D. O. (25. Januar 2020). Rinder aktuell: Kälberdurchfall durch Kryptosporidien – Hartnäckig und weitverbreitet. BAUERNBLATT, S. 52-53.
Losand, B., Falkenberg, U., Krömker, V., Konow, M., & Flor, J. (2. März 2021). Kälberaufzucht in MV – Alles im grünen Bereich? 30. Milchrindtag Mecklemburg-Vorpommern.
Luginbühl, A., K. Reitt, A. Metzler, M. Kollbrunner, L. Corboz, and P. Deplazes. “Feldstudie Zu Prävalenz Und Diagnostik Von Durchfallerregern Beim Neonaten Kalb Im Einzugsgebiet Einer Schweizerischen Nutztierpraxis.” Schweizer Archiv für Tierheilkunde 147, no. 6 (2005): 245–52. https://doi.org/10.1024/0036-7281.147.6.245.
Olias, P., Dettwiler, I., Hemphill, A., Deplazes, P., Steiner, A., & Meylan, M. (2018). Die Bedeutung der Cryptosporidiose für die Kälbergesundheit in der Schweiz. Schweiz Arch Tierheilkd, Band 160, Heft 6, Juni 2018, 363-374.
Santín, M., Trout, J. M., Xiao, L., Zhou, L., Greiner, E., & Fayer, R. (2004). Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Veterinary Parasitology 122, 103-117.
Shaw, H. J., Innes, E. A., Marrison, L. J., Katzer, F., & Wells, B. (2020). Long-term production effects of clinical cryptosporidiosis in neonatal calves. International Journal for Parasitology 50, 371-376.
Silverlås, C., H. Bosaeus-Reineck, K. Näslund, and C. Björkman. “Is There a Need for Improved Cryptosporidium Diagnostics in Swedish Calves?” International Journal for Parasitology 43, no. 2 (2013): 155–61. https://doi.org/10.1016/j.ijpara.2012.10.009.
Thomson, Sarah, Carly A. Hamilton, Jayne C. Hope, Frank Katzer, Neil A. Mabbott, Liam J. Morrison, and Elisabeth A. Innes. “Bovine Cryptosporidiosis: Impact, Host-Parasite Interaction, and Control Strategies.” Veterinary Research 48, no. 1 (2017). https://doi.org/10.1186/s13567-017-0447-0.
Uhde, F., Kaufmann, T., Sager, H., Albini, S., Zanoni, R., & Schelling, E. (2008). Prevalence of four enteropathogens in the feces of young diarrhoeic dairy calves in Switzerland. Veterinary Record (163), 362-366.