Decoding the connection between stress, endotoxins, and poultry health

By Technical Team, EW Nutrition
Stress can be defined as any factor causing disruptions to homeostasis, which triggers a biological response to regain equilibrium. We can distinguish four major types of stressors in the poultry industry:
- Technological: related with management events and conditions
- Nutritional: involving nutritional disbalances, feed quality and feed management
- Pathogenic: comprising health challenges.
- Environmental: changes in environment conditions
In practical poultry production, multiple stress factors occur simultaneously. Their effects are also additive, leading to chronic stress. The animals are not regaining homeostasis and continuously deviate the use of resources through inflammation and the gut barrier-function, thus leading to microbiome alteration. As a consequence, welfare, health, and productivity are compromised.
What are endotoxins?
Bacterial lipopolysaccharides (LPS), also known as endotoxins, are the main components of the outer membrane of all Gram-negative bacteria and are essential for their survival. LPS have direct contact with the bacteria’s surroundings and function as a protection mechanism against the host’s immunological response and chemical attacks from bile salts, lysozymes, or other antimicrobial agents.
Gram-negative bacteria are part of animals’ microbiota; thus, there are always LPS in the intestine. Under optimal conditions, this does not affect the animals, because intestinal epithelial cells are not responsive to LPS when stimulated from the apical side. In stress situations, the intestinal barrier function is impaired, allowing the passage of endotoxins into the blood stream. When LPS are detected by the immune system either in the blood or in the basolateral side of the intestine, inflammation and changes in the gut epithelial structure and functionality occur.
The gut is critically affected by stress
Even when there is no direct injury to the gut, signals from the brain can modify different functions of the intestinal tract, including immunity. Stress can lead to functional disorders, as well as to inflammation and infections of the intestinal tract. Downstream signals act via the brain–gut axis, trigger the formation of reactive oxygen and nitrogen species as well as local inflammatory factors, and circulating cytokines, affecting intestinal homeostasis, microbiome, and barrier integrity.
Stress then results in cell injury, apoptosis, and compromised tight junctions. For this reason, luminal substances, including toxins and pathogens, leak into the bloodstream. Additionally, under stress, the gut microbiome shows and increment on Gram-negative bacteria (GNB). For instance, a study by Minghui Wang and collaborators (2020) found an increase of 24% in GNB and lower richness, in the cecum of pullets subjected to mild heat stress (increase in ambient temperature from 24 to 30°C).
Both these factors, barrier damage and alterations in the microbiome, facilitate the passage of endotoxins into the blood stream, which promotes systemic chronic inflammation.
What categories of stress factors trigger luminal endotoxins’ passage into the bloodstream?
Technological stress
Various management practices and events can be taken as stressors by the animals’ organism. One of the most common examples is stocking density, defined as the number of birds or the total live weight of birds in a fixed space. High levels are associated with stress and loss of performance.
A study from the Chung-Ang University in 2019 found that broilers with a stocking density of 30 birds/m2 presented two times more blood LPS than birds kept at half of this stocking density. Moreover, the body weight of the birds in the high-density group was 200g lower than the birds of the low-density group. The study concluded that high stocking density is a factor that can disrupt the intestinal barrier.
Nutritional stress
The feed supplied to production animals is designed to contribute to express their genetic potential, though some feed components are also continuous inflammatory triggers. Anti-nutritional factors, oxidized lipids, and mycotoxins induce a low-grade inflammatory response.
For instance, when mycotoxins are ingested and absorbed, they trigger stress and impair immunity in animals. Their effects start in gastrointestinal tract and extend from disrupting immunity to impairing the intestinal barrier function, prompting secondary infections. Mycotoxins can increase the risk of endotoxins in several ways:
- By inducing changes in the intestinal microbiota that increase gram-negative bacteria
- By disrupting the intestinal barrier function, allowing endotoxins (as well as other toxins and pathogens) to cross the gut barrier and pass into the bloodstream
- By alterations in the immune response, low doses of mycotoxins, such as trichothecenes, induce the upregulation of pro-inflammatory cytokines. A possible synergy can be inferred as when they are together, the effects may be prolonged and require a lower dosage to be triggered.
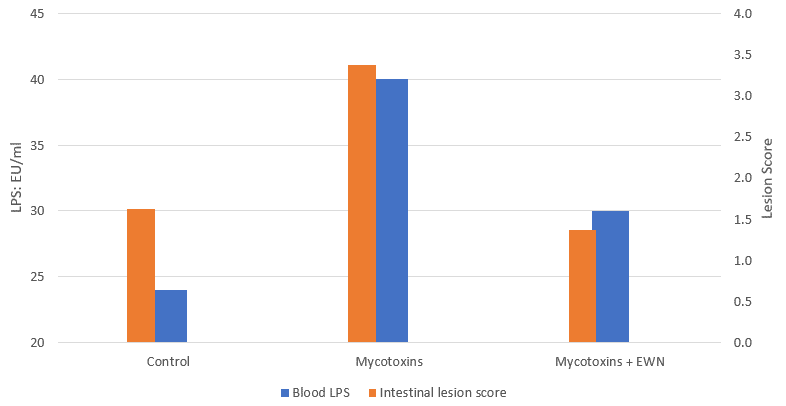
A study conducted by EW Nutrition (Figure 1) shows an increase in intestinal lesions and blood endotoxins after a mycotoxin challenge of 200pbb of Aflatoxin B1 + 360ppb Ochratoxin in broilers at 21 days of age. The challenged birds show two times more lesions and blood endotoxins than the ones in the unchallenged control. The use of the right mitigation strategy, a product based on bentonite, yeast cell walls, and phytogenics (EW Nutrition GmbH) successfully prevented these effects as it not only mitigates mycotoxins, but also targets endotoxins in the gut.
Pathogenic stress
Intestinal disease induces changes in the microbiome, reducing diversity and allowing pathogens to thrive. In clinical and subclinical necrotic enteritis (NE), the intestinal populations of GNB, including Salmonella and E.coli also increases. The lesions associated with the pathogen compromise the epithelial permeability and the intestinal barrier function, resulting in translocation of bacteria and LPS (Figure 5) into the bloodstream and internal organs.
Environmental stress
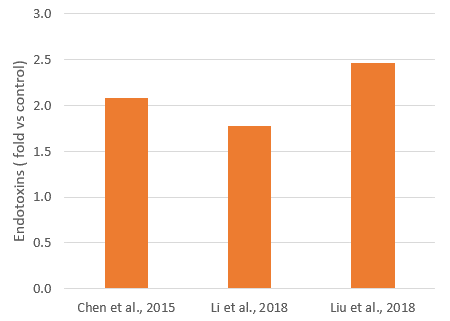
Acute and chronic heat and cold stress increases gut permeability, by increasing intestinal oxidative stress and disrupting the expression of tight junction proteins. This results in the damage and destruction of intestinal cells, inflammation, and imbalance of the microbiota. An increased release and passage of endotoxins has been demonstrated in heat stress (Figure 3), as well as a higher expression of TLR-4 and inflammation.
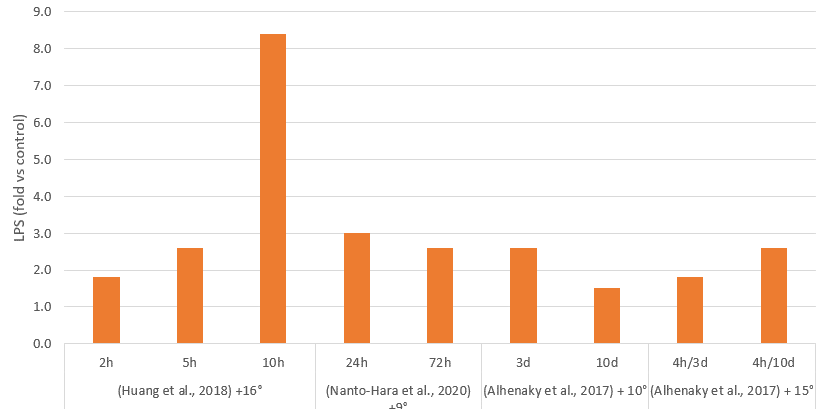
Zhou and collaborators (2021) showed that 72 hours of low temperature treatment in young broilers increased intestinal inflammation and expression of tight junction proteins, while higher blood endotoxins indicate a disruption of the intestinal barrier. As a consequence, the stress decreased body gain and increased the feed conversion rate.
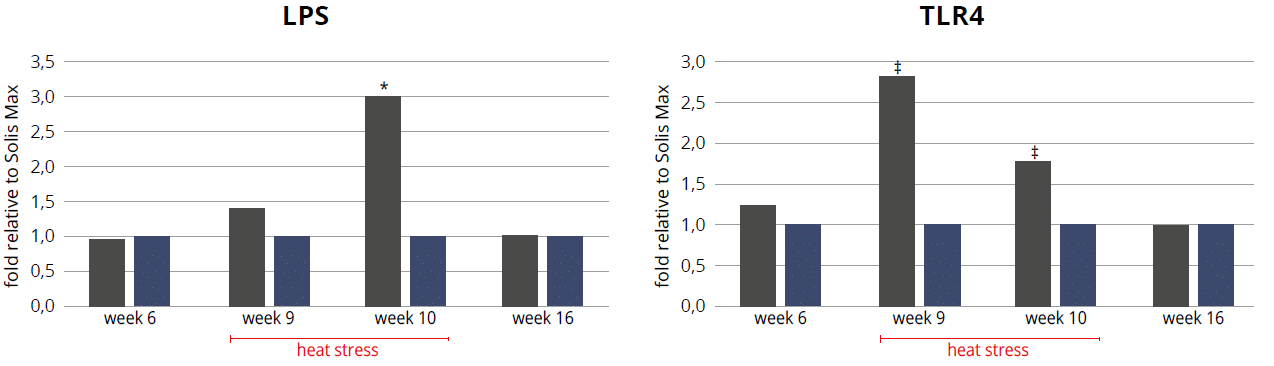
An experiment conducted by EW Nutrition GmbH with the objective of evaluating the ability of a toxin mitigation product to ameliorate heat-stress induced LPS. For the experiment, 1760 Cobb 500 pullets were divided into two groups, and each was placed in 11 pens of 80 hens, in a single house. One of the groups received feed containing 2kg/ton of the product from the first day. From week 8 to week 12, the temperature of the house was raised 10°C for 8 hours every day.
Throughout the heat stress period, blood LPS (Fig 4) was lower in the pullets receiving the product, which allowed lower inflammation, as evidenced by the lower expression of TLR4 (Fig. 5). Oxidative stress was also mitigated with the help of the combination of phytomolecules in the product, obtaining 8.5% improvement on serum total antioxidant capacity (TAC), supported by an increase in in superoxide dismutase (SOD glutathione peroxidase (GSH) and a decrease in malondialdehyde (MDH).
In practice: there is no silver bullet
In commercial poultry production, a myriad stressors may occur at the same time and some factors trigger a chain of events that work to the detriment of animal health and productivity. Reducing the solution to the mitigation of LPS is a deceitfully simplistic approach. However, this should be part of a strategy to achieve better animal health and performance. In fact, EW Nutrition’s toxin mitigation product alone helped the pullets to achieve 3% improvement in body weight and 9 points lower cumulative feed conversion (Figure 6).
Keeping the animals as free of stress as possible is a true priority for poultry producers, as it promotes animal health as well as the integrity and function of the intestinal barrier. Biosecurity, good environment, nutrition and good management practices are crucial; the use of feed additives to reduce the consequences of unavoidable stress also critically supports the profitability of poultry operations.



 A
A