Acidifiers support piglets after weaning
By Dr. Inge Heinzl, Editor, EW Nutrition
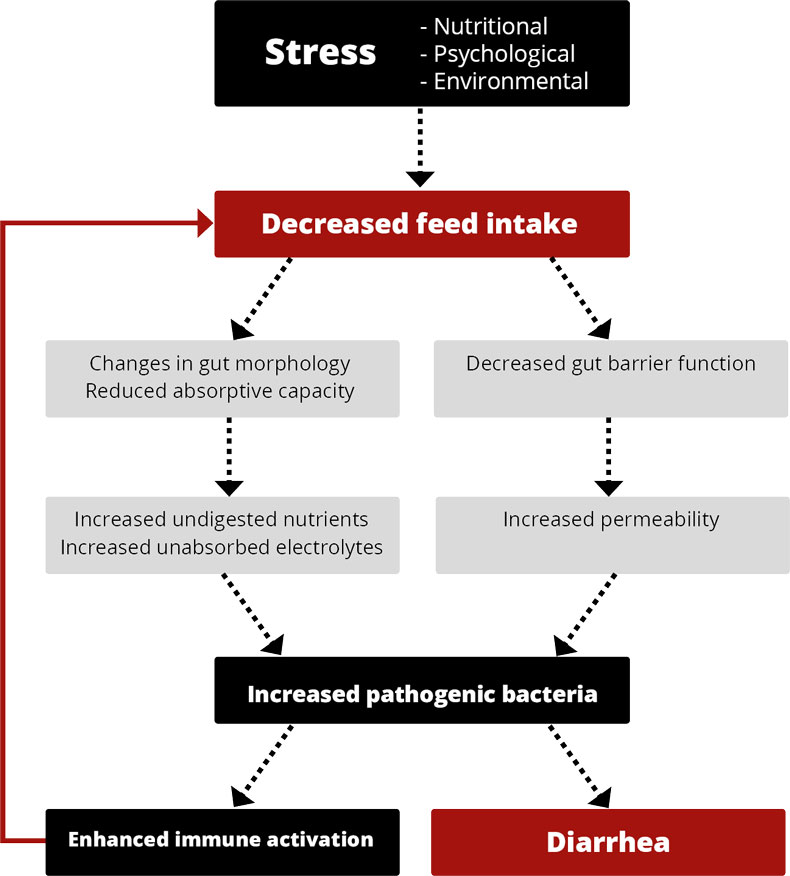
In piglet production, high productivity, meaning high numbers of healthy and well-performing piglets weaned per sow and year, is the primary target. Diarrhea around weaning often gets in the way of achieving this goal.
Up to the ban of antibiotic growth promoters in 2006, antibiotics were often applied prophylactically to help piglets overcome this critical time. Zinc oxide (ZnO) application is another measure that cannot be used anymore to prevent piglet diarrhea. Effective alternatives are required.
Weaning – a critical point in piglets’ life
Weaning stress is well-known to have a negative impact on the balance of the intestinal microflora and gastrointestinal functions (Miller et al., 1985). Suckling piglets have a limited ability to produce hydrochloric acid, but nature has a solution to compensate for this inadequacy. The lactobacilli present in the stomach can use the lactose in the sow’s milk to produce lactic acid (Easter, 1988). In nature, the piglets would start to eat small amounts of solid feed at about three weeks when the sow’s milk production no longer covers their nutrient demand. By increasing the feed intake, the piglets stimulate hydrogen chloride (HCl) production in their stomachs.
In piglet production, where weaning occurs at three or four weeks of age, the piglets are still not eating considerable amounts of solid feed. It is often the case that 50 % of the piglets take feed at the earliest after 24 h, and 10 % accept the first feed only after 48 h (Brooks, 2001). Additionally, hard grains in the diet can physically damage the small intestine wall, reducing villus height and crypt depth (Kim et al., 2005).
Only a minor production of HCl, no more lactose supply for the lactobacilli, varying feed intake, and high buffering capacity of the feed lead to a pH of >5 in the stomach.
The higher stomach pH is partly responsible for problems after weaning
A pH higher than 5, besides causing direct effects on the microflora in the stomach, has consequences for the whole digestive tract and digestion.
A high pH is favorable for certain microorganisms, including coliforms (Sissons, 1989) and other acid-sensitive bacteria such as Salmonella typhimurium, Salmonella typhi, Campylobacter jejuni, and V. cholerae (Smith, 2003).
-
Lower activity of proteolytic enzymes
In the stomach, the conversion of pepsinogen to pepsin, which is responsible for protein digestion, is catalyzed under acid conditions (Sanny et al., 1975). Pepsin works optimally at two pH levels: pH 2 and pH 3.5 (Taylor, 1959). With increasing pH, the activity decreases; at pH 6, it stops. Therefore, a high pH can lead to poor digestion and undigested protein arriving in the intestine. There, it can be used as “feed” for harmful bacteria, leading to their proliferation. Barrow et al. (1977) found higher counts of coliforms in piglets’ intestinal tract two days after weaning, while the number of lactobacilli was depressed.
In the lower parts of the gastrointestinal tract (GIT), the final products of the pepsin protein digestion are needed to stimulate the secretion of pancreatic proteolytic enzymes. If no final products arrive, the enzymes are not activated, and the inadequate protein digestion continues. Additionally, gastric acid is the main stimulant for bicarbonate secretion in the pancreas, neutralizing gastric acid and providing an optimal pH environment for the digestive enzymes working in the duodenum.
-
Expedited digesta transport
The stomach pH also influences the transport of digesta. The acidity of the chyme leaving the stomach and arriving in the small intestine is decisive for the amount of digesta being transferred from the stomach to the small intestine. Acid-sensitive receptors provide feedback regulation to prevent the stomach from emptying until the duodenal chyme can be neutralized by pancreatic or other secretions (Pohl et al., 2008). Therefore, a higher pH in the stomach leads to a faster transport of the digesta, resulting in worse feed digestion.
-
Proliferation of microorganisms
A high pH is favorable for certain microorganisms, including coliforms (Sissons, 1989) and other acid-sensitive bacteria such as Salmonella typhimurium, Salmonella typhi, Campylobacter jejuni, and V. cholerae (Smith, 2003).
Elevated stomach pH + incomplete immune system = diarrhea
Acidifiers can mitigate the adverse effects of weaning on piglets
To overcome this critical time of weaning and maintain performance, acidifiers can be a helpful tool. They improve gut health, stimulate immunity, and serve as nutrient sources – while also positively affecting feed and water hygiene.
What are acidifiers?
Acidifiers’ role in pig nutrition has evolved from feed preservatives to stomach pH stabilizers, compensating for young pigs’ reduced digestive capacity (Ferronato and Prandini, 2020). They are now used to replace antibiotic growth promoters and ZnO, which were applied for a long time to mitigate the negative effects of weaning.
In general, both organic and inorganic acids and their salts feature in animal nutrition. They can be added to the feed or the water.
Organic acids: Commonly used with good results
Feed acidifiers are usually organic acids, including fatty and amino acids. Their carboxyl functional group is responsible for their acidic specificity as feed additives (Pearlin et al.,2019). Their pKa, the pH where 50 % of the acid occurs in a dissociated form, is decisive for their antimicrobial action. In animal nutrition, acids with pKa 3-5 are typically used (Kirchgeßner and Roth, 1991).
Organic acids used as feed additives can be divided into three groups:
- Simple monocarboxylic acids such as formic, acetic, propionic, and butyric acid
- Carboxylic acids with a hydroxyl group such as lactic, malic, tartaric, and citric acid
- Short-chain carboxylic acids with double bonds – fumaric and sorbic acid.
The primary acids for pig nutrition are acetic, fumaric, formic, lactic, benzoic, propionic, sorbic, and citric acids (Roth and Ettle, 2005).
Inorganic acids – the low-cost version
Inorganic acids are cheaper than organic acids, but their only effect is to decrease the pH. Additionally, they are extremely corrosive and dangerous liquids due to their strong acidity in a pure state (Kim et al., 2005).
Salts are easier to handle
The advantage of salts over free acids is that they are generally odorless and easier to handle in the feed manufacturing process due to their solid and less volatile form. Higher solubility in water is a further advantage compared to free acids (Huyghebaert and Van Immerseel, 2011; Roth and Ettle, 2005; Partanen and Mroz, 1999). The better handling and higher palatability make acid salts a more user-friendly method to apply acids to feed and water without compromising their efficacy (Luise et al., 2020).
The salts are mainly produced with calcium, potassium, and sodium. They include calcium formate, potassium diformate, sodium diformate, and sodium fumarate.
Blends
A mixture of diverse acidifiers combines the different characteristics of these substances. Perhaps, there may be synergistic effects. Acid blends are more and more used as feed additives. They have a wider-ranging action than single substances.
Roth et al. (1996) showed that a combination of formic acid with various formats is more effective than the application of formic acid alone.
The main effects of acidifiers
Acidifiers support piglets during the critical time after weaning through different modes of action. The final results are:
- Improvement in gut health
- Increase in growth performance
- Stabilization of the immune system.
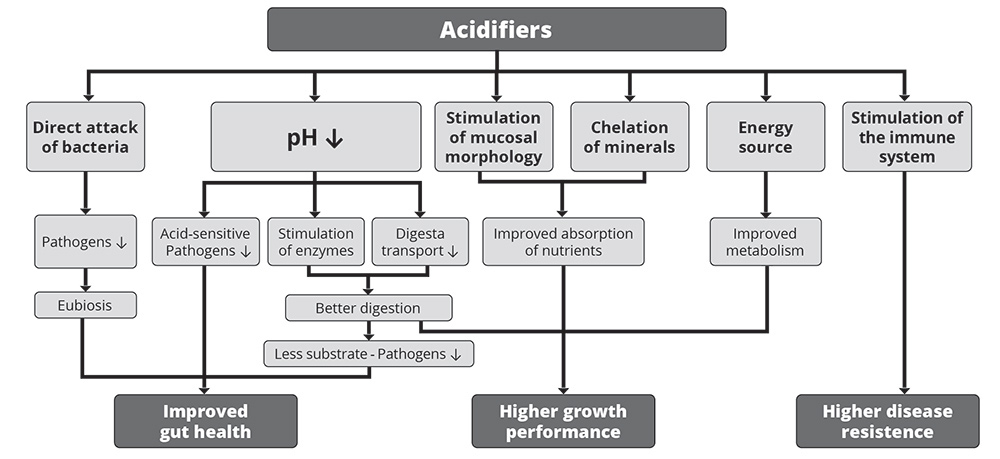
1. Improvement in gut health
As shown in figure 1, the improvement in gut health relies on the antimicrobial effect of organic acids and the decrease in the stomach’s pH.
1.1 Organic acids directly attack bacteria
Organic acids not only act through their pH-decreasing effect but also directly attack pathogens. Due to their lipophilic character, organic acids can pass the bacterial cell membrane when they are in their undissociated form (Partanen in Piva et al., 2001). The lower the external pH, the more undissociated acid can pass the membrane.
Within the cell, the pH is higher. Hence, the organic acid dissociates and releases hydrogen ions, reducing the cytoplasmic pH from alkaline to acid. Cell metabolism is depressed at lower pH. Therefore, the bacterial cell needs to expel protons to get the cytoplasmic pH back to normal. As this is an energy-consuming process, more prolonged exposure to organic acids kills the bacterium. Additionally, the anions staying within the cell disturb the cell’s metabolic processes and participate in killing the bacterium.
Studies from Van Immerseel et al. (2006) revealed that many fermentative bacteria could let their intracellular pH decline and prevent increased acid penetration. Bacteria with a neutrophil pH, however, react more sensitively.
1.1 Decreased pH reduces non-acid-tolerant pathogens
There is a direct effect of pH on the microflora. Some pathogenic bacteria are susceptible to low pH. The proliferation of, e.g., E. coli, Salmonella, and Clostridium perfringens is minimized at a pH<5. Acid-tolerant bacteria such as lactobacilli or bifidobacteria, however, survive. Many lactobacilli can produce hydrogen peroxide, which inhibits, e.g., Staphylococcus aureus or Pseudomonas spp. (Juven and Pierson, 1996).

Already Fuller (1977) showed in in vitro experiments that certain bacteria such as Streptococci, Salmonella, and B. cereus don’t grow in an environment with pH 4.5 or even die (Micrococcus). In contrast, Lactobacilli are not so susceptible to this low pH. Using the same binding sites as harmful bacteria, they suppress coliforms, for example. Kirchgeßner et al. (1997) found a stronger reduction of E. coli than Lactobacilli and Bifidobacteria in different gut segments when exposed to 1.25 % formic acid.
1.2 Recovery of eubiosis through reduction of substrate
The reduction of the pH through organic acids maintains or stimulates the secretion of proteolytic enzymes in the stomach (pepsin) and pancreatic enzymes. Additionally, the acid leaving the stomach is partly responsible for regulating gastric emptying (Ravindran and Kornegay, 1993; Mayer, 1994). Both effects by improving protein digestion, reduce the fermentable substrates arriving in the hindgut. This decreases the quantity of fermentable substrate arriving in the intestine and, therefore, the growth of undesired pathogens.
2. Promotion of growth
2.1 Enhanced digestion of macronutrients
As explained above, the acidity in the stomach is responsible for the activation and stimulation of enzymes. Additionally, the lower pH keeps the feed in the stomach for longer. Both result in better digestion.
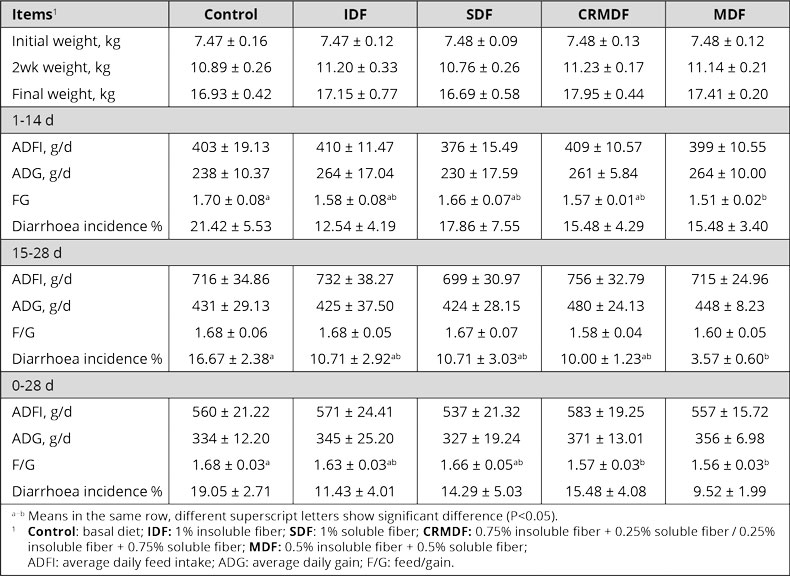
The improved utilization of nutrients leads to higher daily gain and better feed conversion. In pigs, the growth-promoting effect of organic acids is particularly pronounced during the first few weeks after weaning (Roth and Ettle, 2005). Some examples of the growth-promoting effect of formic and propionic acid feature in table 1.
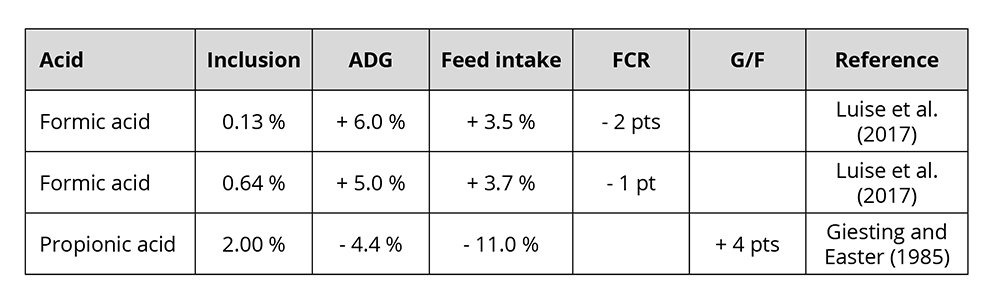 Table 1: Influence of two commonly used organic acids in animals on growth performance
Table 1: Influence of two commonly used organic acids in animals on growth performance
Varying results are mainly due to the character of the organic acid, the dosage, the buffering capacity, and the possible reduction of feed intake in case of a high dosage (Roth and Ettle, 2005).
2.2 Improved utilization of minerals
Minerals are essential for metabolic processes and, thus, healthy growth. Chelated minerals show a higher digestibility. Acidic anions of the acidifiers form complexes (chelates) with cationic minerals such as Ca, Zn, P, and Mg. The resulting higher digestibility and absorption lead to decreased excretion of supplemented minerals and, therefore, to a lower environmental burden. Kirchgeßner and Roth (1982), e.g., reported an improved absorption and retention of Ca, P, and Zn with the addition of fumaric acid. However, there are also trials showing no effect of acidification of the diet on mineral balance (Radecki et al., 1988).
 Phytic acid
Phytic acid
Another factor influencing the absorption of minerals, mainly phosphorus, is the amount of intrinsic or microbial phytase in the diet (Rutherfurd et al., 2012). The enzyme phytase releases phosphorus out of phytic acid and increases its bioavailability. Partanen and Mroz (1999) showed that organic acids improve the performance of phytase and, therefore, the bioavailability of phosphorus in the diet.
Besides a better utilization by the animal, improved absorption of minerals means preserving the environment and direct cost-saving, as mineral supplements are expensive.
2.3 Stimulation of gut and stomach mucosal morphology
An intact gut mucosa with a preferably high surface is vital for efficient nutrient absorption. Many trials show that organic acids improve the condition of the mucosa:
Organic acids stimulate cell proliferation
In an in vitro trial with pig hindgut mucosa, butyric acid stimulated epithelial cell proliferation in a dose-dependent manner (Sakata et al., 1995).
Blank et al. reported that fumaric acid, being a readily available energy source, may have a local trophic effect on the small intestines’ mucosa. Due to faster recovery of the gastrointestinal epithelial cells after weaning, this trophic effect may increase the absorptive surface and digestive capacity in the small intestines.
Organic acids influence villi length and crypt depth in the gut
Ferrara et al. (2016) observed a trend toward longer villi with a mixture of short-chain organic acids and mid-chain fatty acids, compared to the negative control.
The addition of Na-butyrate to the feed leads to increased crypt depth, villi length, and mucosa thickness in the distal jejunum and ileum, according to Kotunia et al. (2004). However, the villi length and mucosa thickness were reduced in the duodenum.
According to Gálfi and Bokori (1990), a diet with 0.17% sodium butyrate increased the length of ileal microvilli and the depth of caecal crypts in pigs weighing between 7 and 102 kg.
Organic acids strengthen stomach mucosa
Mazzoni et al. (2008) reported that sodium butyrate applied orally at a low dose influenced gastric morphology and function (thickening the mucosa), presumably due to its action on mucosal maturation and differentiation.
2.4 Pigs can use organic acids as an energy source
Organic acids are usually added to the feed in small doses. As some organic acids are intermediary products of the citric acid cycle, they are an energy source after being absorbed through the gut epithelium by passive diffusion. Their gross energy can be fully metabolized (Pearlin et al., 2019; Roth and Ettle, 2005; Suiryanrayna and Ramana, 2015).
The gross energy supply varies according to the acid. Roth and Ettle (2005) determined values between 6 kJ/g (formic acid) and 27 kJ/g (sorbic acid). Pearlin et al. (2019) calculated that 1 M of fumaric acid generates 1.340 kJ or 18 M ATP; this is comparable to the energy provision of glucose. Citric acid’s energy provision is similar; acetic and propionic acid require 18 and 15 % more energy to generate 1 M ATP.
Acidifiers improve immune response
The immune system, especially at the sensitive life stage of weaning, plays an essential role for the piglet. Acidifiers have been shown to stimulate or support the immune system. Ahmed et al. (2014) showed that citric acid (0.5 %) and a blend of acidifiers composed of formic, propionic, lactic, phosphoric acid + SO2(0.4 %) significantly increased the level of serum IgG. IgG are part of the humoral immune system. They mark foreign substances to be eliminated by other defense systems.

In a trial conducted by Ren et al. (2019), piglets receiving a mixture of formic and propionic acid showed lower concentrations of plasma tumor necrosis factor-α, regulating the activity of diverse immune cells. Furthermore, interferon-γ and interleukin (Il)-1ß were lower than in the control group after the challenge with E. coli (ETEC). In this trial, the addition of organic acids to the feed alleviated the inflammatory response in a way comparable to antibiotics.
In a nutshell
Organic acids are no longer seen as pure acidifiers but as growth promoters and potential antibiotic substitutes due to their positive effect on the gastrointestinal tract. Their main effect, the decrease of pH, entails consequences from inhibiting pathogenic bacteria and improved digestion to enhanced health and growth.
Research indicates that acidifiers can be a viable alternative to antibiotic growth promoters and ZnO for ensuring healthy piglet production after weaning.