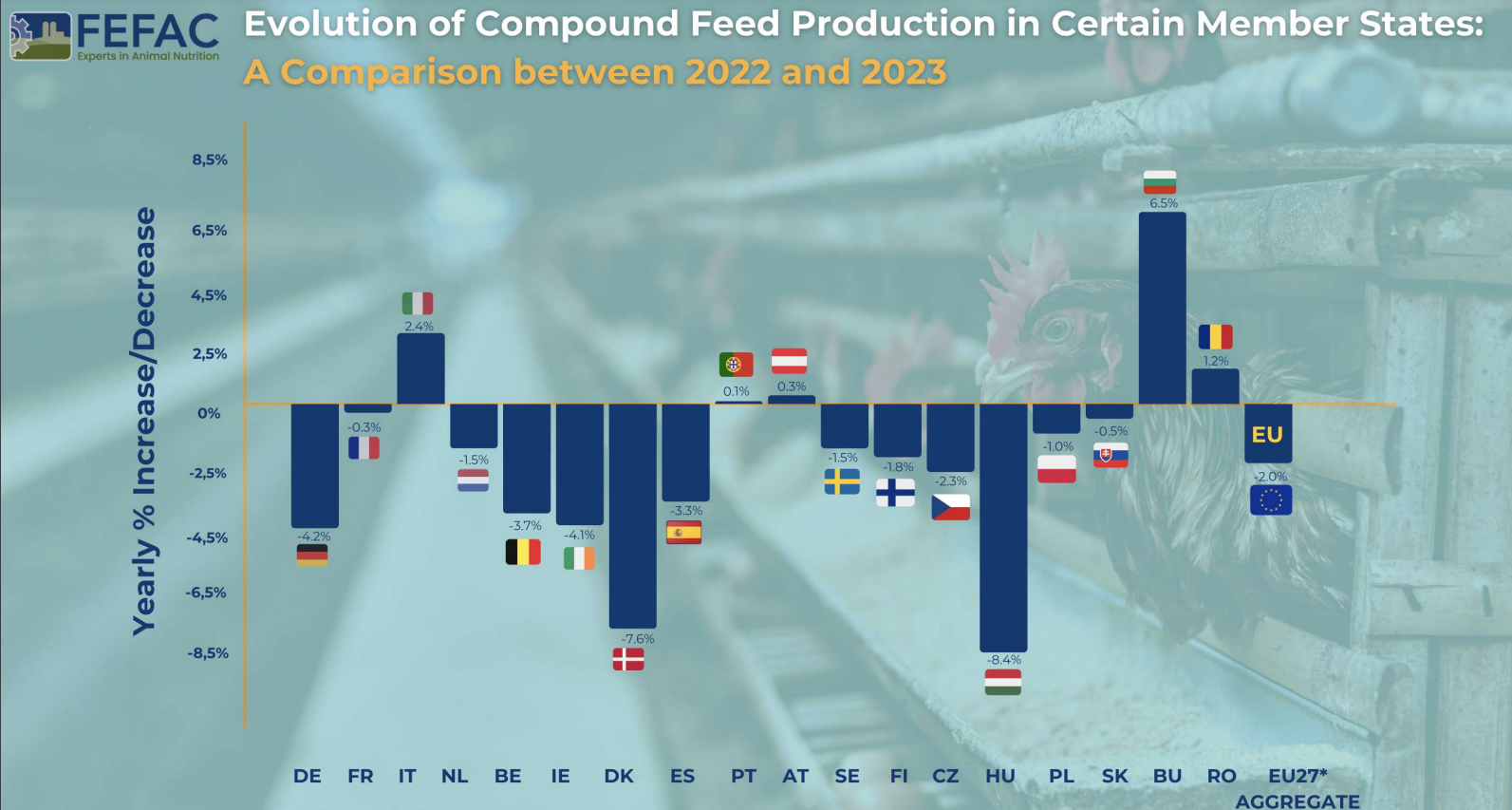
Mycotoxins in poultry – External signs can give a hint

Part 1: Impact on Feathering
By Dr. Inge Heinzl, Editor, EW Nutrition
Mycotoxins are known to decrease health and performance in poultry production. Their modes of action, such as reducing protein synthesis and promoting oxidative stress and apoptosis, lead to cell destruction and lower cell replacement, affecting several organs and tissues.
When different stress factors collude, such as high temperatures and humidity, poor ventilation, high stocking density, and management events, the effects of in-feed mycotoxins can reach a higher level, which may include external signs.
The most common and recognized external sign of mycotoxicosis is mouth lesions caused by trichothecenes, which are highly associated with the presence of T-2 in the feed. However, other signs may appear, such as paleness of combs, shanks, and feet, as well as leg problems, ruffled feathers and poor feather coverage, feed passage, and abnormal feces.
In a series of articles, we want to report on external signs facilitating a differential diagnosis of mycotoxin contamination. This is necessarily followed by feed or raw material mycotoxin analysis and strategies to avoid or mitigate the effects of mycotoxin contamination in poultry production. In the first article, we will cover feathers.
A healthy plumage is crucial for growth and reproduction
Feathering is a crucial aspect of poultry health and productivity. Feathers are essential for thermoregulation, locomotion, adequate skin protection, and reproductive success, protecting hens from injury during mating. Inadequate feathering can lead to lower feed efficiency (Leeson and Walsh, 2004) as well as loss in fertility and chick production (Fisher, 2016). Mycotoxins in poultry feed can compromise feather quality in poultry production animals. This first article delves into the relationship between mycotoxins and poor feathering, exploring different mycotoxins and their mechanisms of action.
In which way do mycotoxins compromise feathering?
On the one hand, chronic mycotoxin exposure impairs the digestive process, hindering the absorption and utilization of vital nutrients essential for feather growth. This disruption can lead to malnutrition, directly impacting the quality and health of feathers. On the other hand, mycotoxins also interfere with metabolic processes critical for feather development, such as keratin synthesis (Wyatt et al., 1975; Nguansangiam, 2004). Enzymatic pathways involved in synthesizing keratin, the protein building block of feathers, are particularly vulnerable to mycotoxin-induced disruptions. The presence of mycotoxins in feed has been associated with the manifestation of sparse feathering and the sticking out of feathers at an unnatural angle (Emous and Krimpen, 2019). In the case of multiple mycotoxins occurring in the feed, even at singularly unimportant concentrations, a negative impact on feathering is possible. Different mycotoxins have different target organs and consequences for the animal, so their ways of compromising feathering also vary. As feathering needs protein availability, all mycotoxins affecting the protein metabolism or the absorption of nutrients also impact the feathering process. Let us look at the most prominent mycotoxins.
1. T-2 toxin
Due to climate change, T-2 toxins are on the rise. In the US, more than 50% of the tested samples contained T-2 toxin; in Europe, we found it in 31%, and in China, in 82% of the samples (EW Nutrition, 2024). The highest level was found in Europe, with 850 ppb.
Adverse effects of T-2 toxin in goslings were shown by Gu et al. (2023), who exposed the animals to 6 different levels of T-2 toxin, from 0.2 to 2.0 mg T-2 toxin/kg of feed. The goslings showed a sparse covering with short, dry, rough, curly, and gloss-free feathers on their back with dosages ≥0.8 mg/kg. When zooming on, T-2 can cause necroses of the layer of regenerative cells in the feather base, implying malformation or absence of new feathers, as well as structural damage to existing feathers on the base of the ramus and barb ridges (Hoerr et al. (1982), Leeson et al. (1995)).
The effects in feather regenerative cells are dose-dependent, as confirmed by Hoerr et al. (1982), who applied different doses of T-2 toxin (1.5, 2, 2.5, and 3 mg/kg body weight/day) to 7-day-old broilers for 14 days. Delayed feather development, especially at high dosages, was noticed, as well as malformations and opaque bands in the feathers, the latter probably caused by a segmental reduction in diameter.
Manafi et al. (2015) noticed feather malformations when broiler chickens were challenged with 0.5 ppm T-2 toxin in the feed in combination with an inoculation of 2.4×108 cfu Mycoplasma gallisepticum. When the chickens were challenged only with T-2 toxin, the feathers were ruffled, showing that a coincidence of stress factors even aggravates the symptoms.
2. Aflatoxins
Aflatoxins, produced by certain Aspergillus species, are among the most notorious mycotoxins. Looking at test results of the last year, Aflatoxin shows incidences between 25 (USA) over 40-65% (Europe, LATAM, MEA, and SEAP) up to 84-88% (China and South Asia) with average levels up to 42 ppb in South Asia (EW Nutrition, 2023). However, more information about the concrete impact of aflatoxins on feathering is needed. They may indirectly affect feathering because they impact digestion and the utilization of nutrients or trace minerals such as zinc, which is essential for the feather construction process. Damage to the liver impacts protein metabolism, and keratin is also necessary for feather production.
In other studies, Muhammad et al. (2017) fed 5 mg AFB1/kg to Arbor Acres broilers, and the birds showed ruffled feathers. A significantly lower feather shine was noticed by Saleemi et al. (2020) when they gave the animals 300 μg AFB1/kg of feed, and the birds of Zafar et al. (2017) showed ruffled, broken, dull, and dirty feathers after six weeks of feeding an aflatoxin-contaminated diet.
3. Ochratoxin
Ochratoxins, commonly produced by Aspergillus and Penicillium fungi, also pose a significant threat to poultry. When looking at the mycotoxin report, this mycotoxin was found in 16% (Europe) to 70% (SEAP) of the samples (EW Nutrition, 2023). Ochratoxins primarily affect feathering by compromising the structural integrity of feathers and causing delayed feathering in broilers (Leeson, 2021).
Several trials have shown the negative impact of ochratoxin on feather quality. Hassan et al. (2010) fed OTA to laying hens and saw a dose-dependent (dosages from 0 to 10 mg/kg feed) occurrence of ruffled and broken feathers in the OTA group, whereas the plumage of the control group was shiny and well-formed. Hameed et al. (2012) also realized dull feathers when feeding 0.4 and 0.8 mg OTA per kg of feed. A further dose-dependent decrease in feather quality was described by Khan et al. (2023) in broiler chicks. He injected them with dosages from 0.1 to 1.7 mg/kg body weight on day 5 of age and saw a deterioration of feather appearance (rippled feathers) in the groups with the higher dosages of 1.3 and 1.7 mg/kg. Abidin et al. (2016) observed a similar dose-dependent deterioration of the feather quality in white Leghorn cockerels when feeding 1 or 2mg OTA/kg feed.
Combinations of aflatoxins and ochratoxins were also tested. Khan et al. (2017) fed moldy feed naturally containing 56 µg OTA and 136 µg AFB1 per kg to layer hens and saw a deterioration of feather quality with increasing feeding time. Qubih (2017) noticed ruffled feathers when feeding a diet naturally contaminated with 800 ppb of OTA and 100 ppb of AFB1.
4. Scirpenol mycotoxins
Parkhurst et al. (1992) examined the effects of different scirpenol mycotoxins. After feeding graded levels of fusarium mycotoxins to broiler chicks until three weeks of age, they discovered that the impact of scirpenols stretched across the entire feathered body parts and that the degree of feather alteration is dose-dependent. The main alteration was a frayed or even missing web on the medial side of the outer end of the feather due to poor development of the barbs, barbules, and barbicels, and the tip of the feathers became square instead of rounded—the thinner and weaker shafts of the feathers inclined to show an accentuated medial curve.
 Parkhurst et al. (1992)
Parkhurst et al. (1992)
Figure 1: Feathering affected by scirpenol mycotoxins
In their trial, Parkhurst and Hamilton realized that 15-monoacetoxyscirpenol (15-MAS) caused the most severe alterations of feathers, and they determined a minimum effective dose (MED) of 0.5 µg/g diet. The MEDs for 4,15-diacetoxyscirpenol (4,15-DAS) and 3,4,15-triacetoxyscirpenol (TAS) were higher, 2 µg/g and > 8 µg/g, respectively.
How can we enable adequate feathering in poultry?
Adequate feathering of poultry is necessary for the animal’s health and welfare and to ensure fertility and productivity. The occurrence of mycotoxins in the feed – and the probability is high! – can cause poor feathering or the development of malformed feathers.
To best equip broilers, layers, and breeders, their feed must contain all nutrients essential for healthy growth and appropriate feathering. As the risk of contamination of the feed materials is very high (see EW Nutrition’s mycotoxin report 2023), it is of crucial importance to have an efficient mycotoxin risk management in place, which includes sampling, analysis of samples, and the use of mycotoxin binders. EW Nutrition offers MasterRisk, an online tool where farmers and feed millers can feed the results of their feed analysis concerning mycotoxins and get a risk management recommendation.
In the next part of the series, we will report on beak lesions and skin paleness, two other external signs of mycotoxin contamination.
References:
Abidin, Zain ul, Muhammad Zargham Khan, Aisha Khatoon, Muhammad Kashif Saleemi, and Ahrar Khan. “Protective Effects Ofl-Carnitine upon Toxicopathological Alterations Induced by Ochratoxin A in White Leghorn Cockerels.” Toxin Reviews 35, no. 3–4 (August 22, 2016): 157–64. https://doi.org/10.1080/15569543.2016.1219374.
Emous, R. A., and M. M. Krimpen. “Effects of Nutritional Interventions on Feathering of Poultry – a Review.” Poultry Feathers and Skin: The Poultry Integument in Health and Welfare, 2019, 133–50. https://doi.org/10.1079/9781786395115.0133.
Fisher, Colin. “Feathering in Broiler Breeder Females – Aviagen.” https://aviagen.com/, 2016. http://en.aviagen.com/assets/Tech_Center/Broiler_Breeder_Tech_Articles/English/Feathering-in-Broiler-Breeeder-Females-EN-2016.pdf.
Gu, Wang, Qiang Bao, Kaiqi Weng, Jinlu Liu, Shuwen Luo, Jianzhou Chen, Zheng Li, et al. “Effects of T-2 Toxin on Growth Performance, Feather Quality, Tibia Development and Blood Parameters in Yangzhou Goslings.” Poultry Science 102, no. 2 (February 2023): 102382. https://doi.org/10.1016/j.psj.2022.102382.
Hameed, Muhammad Raza, Muhammad Khan, Ahrar Khan, and Ijaz Javed. “Ochratoxin Induced Pathological Alterations in Broiler Chicks: Effect of Dose and Duration.” Pakistan Veterinary Journal Pakistan Veterinary Journal 8318, no. 2 (December 2012): 2074–7764.
Hassan, Zahoor-Ul, M. Zargham Khan, Ahrar Khan, and Ijaz Javed. “Pathological Responses of White Leghorn Breeder Hens Kept on Ochratoxin A Contaminated Feed.” Pakistan Veterinary Journal 30, no. 2 (2010): 118–23.
Hoerr, F. J., W. W. Carlton, and B. Yagen. “Mycotoxicosis Caused by a Single Dose of T-2 Toxin or Diacetoxyscirpenol in Broiler Chickens.” Veterinary Pathology 18, no. 5 (September 1981): 652–64. https://doi.org/10.1177/030098588101800510.
Hoerr, F.J., W.W. Carlton, B. Yagen, and A.Z. Joffe. “Mycotoxicosis Produced in Broiler Chickens by Multiple Doses of Either T‐2 Toxin or Diacetoxyscirpenol.” Avian Pathology 11, no. 3 (January 1982): 369–83. https://doi.org/10.1080/03079458208436112.
Khan, Ahrar, Muhammad Mustjab Aalim, M. Zargham Khan, M. Kashif Saleemi, Cheng He, M. Noman Naseem, and Aisha Khatoon. “Does Distillery Yeast Sludge Ameliorate Moldy Feed Toxic Effects in White Leghorn Hens?” Toxin Reviews, January 25, 2017, 1–8. https://doi.org/10.1080/15569543.2017.1278707.
Khan, Shahzad Akbar, Eiko N. Itano, Anum Urooj, and Kashif Awan. “Ochratoxin-a Induced Pathological Changes in Broiler Chicks.” Pure and Applied Biology 12, no. 4 (December 10, 2023): 1608–16. https://doi.org/10.19045/bspab.2023.120162.
Leeson, S., and T. Walsh. “Feathering in Commercial Poultry II. Factors Influencing Feather Growth and Feather Loss.” World’s Poultry Science Journal 60, no. 1 (March 1, 2004): 52–63. https://doi.org/10.1079/wps20045.
Leeson, Steve. “Effects of Nutrition on Feathering.” Poultry World, May 22, 2021. https://www.poultryworld.net/specials/effects-of-nutrition-on-feathering/.
Leeson, Steven, Gonzalo J. Diaz Gonzalez, and John D. Summers. Poultry metabolic disorders and Mycotoxins. Guelph, Ontario, Canada: University Books, 1995.
Manafi, M., N. Pirany, M. Noor Ali, M. Hedayati, S. Khalaji, and M. Yari. “Experimental Pathology of T-2 Toxicosis and Mycoplasma Infection on Performance and Hepatic Functions of Broiler Chickens.” Poultry Science 94, no. 7 (July 2015): 1483–92. https://doi.org/10.3382/ps/pev115.
Muhammad, Ishfaq, Xiaoqi Sun, He Wang, Wei Li, Xinghe Wang, Ping Cheng, Sihong Li, Xiuying Zhang, and Sattar Hamid. “Curcumin Successfully Inhibited the Computationally Identified CYP2A6 Enzyme-Mediated Bioactivation of Aflatoxin B1 in Arbor Acres Broiler.” Frontiers in Pharmacology 8 (March 21, 2017). https://doi.org/10.3389/fphar.2017.00143.
Nguansangiam, Sudarat, Subhkij Angsubhakorn, Sutatip Bhamarapravati, and Apichart Suksamrarn. The Southeast Asian J of Tropical Medicine 34, no. 4 (2004): 899–905.
Parkhurst, Carmen R., Pat B. HamiltonON, and Adedamola A. AdemoyeroERO. “Abnormal Feathering of Chicks Caused by Scirpenol Mycotoxins Differing in Degree of Acetylation.” Poultry Science 71, no. 5 (May 1992): 833–37. https://doi.org/10.3382/ps.0710833.
Qubih, T. S. “Relationship between Mycotoxicosis and Calcium during Preproduction Period in Layers.” Iraqi Journal of Veterinary Sciences 26, no. 1 (June 28, 2012): 11–14. https://doi.org/10.33899/ijvs.2012.46888.
Saleemi, M. Kashif, Kamran Ashraf, S. Tehseen Gul, M. Noman Naseem, M. Sohail Sajid, Mashkoor Mohsin, Cheng He, Muhammad Zubair, and Ahrar Khan. “Toxicopathological Effects of Feeding Aflatoxins B1 in Broilers and Its Amelioration with Indigenous Mycotoxin Binder.” Ecotoxicology and Environmental Safety 187 (January 2020): 109712. https://doi.org/10.1016/j.ecoenv.2019.109712.
Wyatt, R.D., P.B. Hamilton, and H.R. Burmeister. “Altered Feathering of Chicks Caused by T-2 Toxin.” Poultry Science 54, no. 4 (July 1975): 1042–45. https://doi.org/10.3382/ps.0541042.
Zafar, Roheena, Farhat Ali Khan, and Muhammad Zahoor. “In Vivo Amelioration of Aflatoxin B1 in Broiler Chicks by Magnetic Carbon Nanocomposite.” Pesquisa Veterinária Brasileira 37, no. 11 (November 2017): 1213–19. https://doi.org/10.1590/s0100-736×2017001100005.